Comparative protein structure modeling using Modeller
- PMID: 18428767
- PMCID: PMC4186674
- DOI: 10.1002/0471250953.bi0506s15
Comparative protein structure modeling using Modeller
Abstract
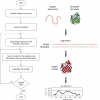
Functional characterization of a protein sequence is one of the most frequent problems in biology. This task is usually facilitated by accurate three-dimensional (3-D) structure of the studied protein. In the absence of an experimentally determined structure, comparative or homology modeling can sometimes provide a useful 3-D model for a protein that is related to at least one known protein structure. Comparative modeling predicts the 3-D structure of a given protein sequence (target) based primarily on its alignment to one or more proteins of known structure (templates). The prediction process consists of fold assignment, target-template alignment, model building, and model evaluation. This unit describes how to calculate comparative models using the program MODELLER and discusses all four steps of comparative modeling, frequently observed errors, and some applications. Modeling lactate dehydrogenase from Trichomonas vaginalis (TvLDH) is described as an example. The download and installation of the MODELLER software is also described.
Figures





References
-
- Abagyan R, Totrov M. Biased probability Monte Carlo conformational searches and electrostatic calculations for peptides and proteins. J. Mol. Biol. 1994;235:983–1002. - PubMed
-
- Arnold K, Bordoli L, Kopp J, Schwede T. The SWISS-MODEL workspace: a web-based environment for protein structure homology modelling. Bioinformatics. 2006;22:195–201. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

