Overview of protein structural and functional folds
- PMID: 18429251
- PMCID: PMC7162418
- DOI: 10.1002/0471140864.ps1701s35
Overview of protein structural and functional folds
Abstract
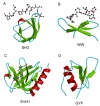
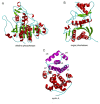
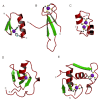
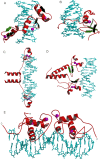
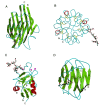
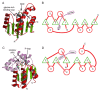
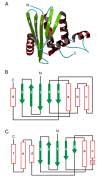
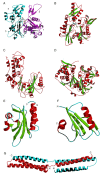
This overview provides an illustrated, comprehensive survey of some commonly observed protein-fold families and structural motifs, chosen for their functional significance. It opens with descriptions and definitions of the various elements of protein structure and associated terminology. Following is an introduction into web-based structural bioinformatics that includes surveys of interactive web servers for protein fold or domain annotation, protein-structure databases, protein-structure-classification databases, structural alignments of proteins, and molecular graphics programs available for personal computers. The rest of the overview describes selected families of protein folds in terms of their secondary, tertiary, and quaternary structural arrangements, including ribbon-diagram examples, tables of representative structures with references, and brief explanations pointing out their respective biological and functional significance.
Figures


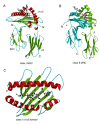


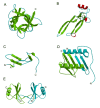
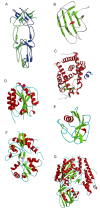
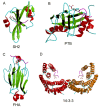





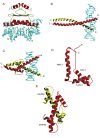


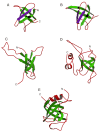
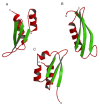
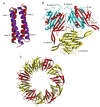







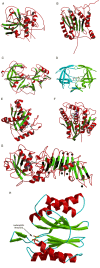
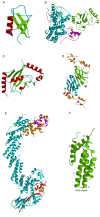
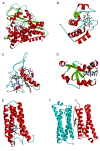
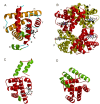
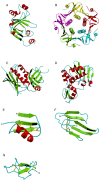

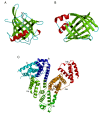

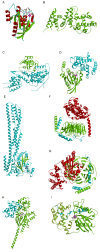



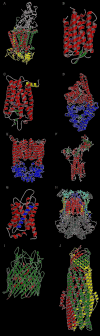
Similar articles
-
Evaluation of protein fold comparison servers.Proteins. 2004 Feb 1;54(2):260-70. doi: 10.1002/prot.10553. Proteins. 2004. PMID: 14696188
-
A consensus view of fold space: combining SCOP, CATH, and the Dali Domain Dictionary.Protein Sci. 2003 Oct;12(10):2150-60. doi: 10.1110/ps.0306803. Protein Sci. 2003. PMID: 14500873 Free PMC article.
-
A comprehensive update of the sequence and structure classification of kinases.BMC Struct Biol. 2005 Mar 16;5:6. doi: 10.1186/1472-6807-5-6. BMC Struct Biol. 2005. PMID: 15771780 Free PMC article.
-
IgStrand: A universal residue numbering scheme for the immunoglobulin-fold (Ig-fold) to study Ig-proteomes and Ig-interactomes.PLoS Comput Biol. 2025 Apr 14;21(4):e1012813. doi: 10.1371/journal.pcbi.1012813. eCollection 2025 Apr. PLoS Comput Biol. 2025. PMID: 40228037 Free PMC article. Review.
-
Understanding the cell in terms of structure and function: insights from structural genomics.Curr Opin Biotechnol. 2006 Oct;17(5):457-64. doi: 10.1016/j.copbio.2006.07.004. Epub 2006 Aug 4. Curr Opin Biotechnol. 2006. PMID: 16890423 Review.
Cited by
-
Genome-wide identification and expression analysis of phosphate-sensing SPX proteins in oats.Front Genet. 2024 Nov 20;15:1469704. doi: 10.3389/fgene.2024.1469704. eCollection 2024. Front Genet. 2024. PMID: 39634271 Free PMC article.
-
Biochemical analysis of Hyalomma dromedarii salivary glands and gut tissues using SR-FTIR micro-spectroscopy.Sci Rep. 2024 Apr 12;14(1):8515. doi: 10.1038/s41598-024-59165-6. Sci Rep. 2024. PMID: 38609442 Free PMC article.
-
Conformational landscapes of rigid and flexible molecules explored with variable temperature ion mobility-mass spectrometry.Nat Commun. 2025 May 6;16(1):4183. doi: 10.1038/s41467-025-59065-x. Nat Commun. 2025. PMID: 40324998 Free PMC article.
-
A Synopsis of Proteins and Their Purification.Methods Mol Biol. 2023;2699:1-14. doi: 10.1007/978-1-0716-3362-5_1. Methods Mol Biol. 2023. PMID: 37646990
-
Molecular Modeling Unveils the Effective Interaction of B-RAF Inhibitors with Rare B-RAF Insertion Variants.Int J Mol Sci. 2023 Jul 31;24(15):12285. doi: 10.3390/ijms241512285. Int J Mol Sci. 2023. PMID: 37569660 Free PMC article.
References
-
- Abrahams, J.P. , Leslie, A.G. , Lutter, R. , and Walker, J.E. 1994. Structure at 2.8 Å resolution of F1‐ATPase from bovine heart mitochondria. Nature 370:621‐628. - PubMed
-
- Adams, M.J. , Ford, G.C. , Koekoek, R. , Lentz, P.J. , McPherson, A. Jr. , Rossmann, M.G. , Smiley, I.E. , Schevitz, R.W. , and Wonacott, A.J. 1970. Structure of lactate dehydrogenase at 2‐8 Å resolution. Nature 227:1098‐1103. - PubMed
-
- Aggarwal, A.K. , Rodgers, D.W. , Drottar, M. , Ptashne, M. , and Harrison, S.C. 1988. Recognition of a DNA operator by the repressor of phage 434: A view at high resolution. Science 242:899‐907. - PubMed
-
- Albert, A. , Yenush, L. , Gil‐Mascarell, M.R. , Rodriguez, P.L. , Patel, S. , Martinez‐Ripoll, M. , Blundell, T.L. , and Serrano, R. 2000. X‐ray structure of yeast Hal2p, a major target of lithium and sodium toxicity, and identification of framework interactions determining cation sensitivity. J. Mol. Biol. 295:927‐938. - PubMed
-
- Alden, R.A. , Birktoft, J.J. , Kraut, J. , Robertus, J.D. , and Wright, C.S. 1971. Atomic coordinates for subtilisin BPN′ (or Novo). Biochem. Biophys. Res. Commun. 45:337‐344. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

