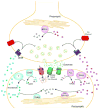
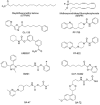
Enzymatic pathways that regulate endocannabinoid signaling in the nervous system
- PMID: 18429637
- PMCID: PMC3150828
- DOI: 10.1021/cr0782067
Enzymatic pathways that regulate endocannabinoid signaling in the nervous system
Figures

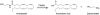


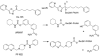


References
-
- Siegel GJ, Agranoff BW, Albers RW, Molinoff PB. Basic Neurochemistry. Raven Press; New York: 1994.
-
- Mechoulam R. The Pharmacohistory of Cannabis sativa. CRC Press; Boca Raton, FL: 1986.
-
- Mackie K. Annu Rev Pharmacol Toxicol. 2006;46:101. - PubMed
-
- Ledent C, Valverde O, Cossu G, Petitet F, Aubert JF, B F, Bohme GA, Imperato A, Pedrazzini T, Roques BP, Vassart G, Fratta W, Parmentier M. Science. 1999;283:401. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

