Selective delivery of beta cell antigen to dendritic cells in vivo leads to deletion and tolerance of autoreactive CD8+ T cells in NOD mice
- PMID: 18430797
- PMCID: PMC2359791
- DOI: 10.1073/pnas.0802644105
Selective delivery of beta cell antigen to dendritic cells in vivo leads to deletion and tolerance of autoreactive CD8+ T cells in NOD mice
Abstract
Type 1 diabetes (T1D) is an autoimmune disease resulting from defects in central and peripheral tolerance and characterized by T cell-mediated destruction of islet beta cells. Cytotoxic CD8(+) T cells, reactive to beta cell antigens, are required for T1D development in the NOD mouse model of the disease, and CD8(+) T cells specific for beta cell antigens can be detected in the peripheral blood of T1D patients. It has been evident that in nonautoimmune-prone mice, dendritic cells (DCs) present model antigens in a tolerogenic manner in the steady state, e.g., in the absence of infection, and cause T cells to proliferate initially but then to be deleted or rendered unresponsive. However, this fundamental concept has not been evaluated in the setting of a spontaneous autoimmune disease. To do so, we delivered a mimotope peptide, recognized by the diabetogenic CD8(+) T cell clone AI4, to DCs in NOD mice via the endocytic receptor DEC-205. Proliferation of transferred antigen-specific T cells was initially observed, but this was followed by deletion. Tolerance was achieved because rechallenge of mice with the mimotope peptide in adjuvant did not induce an immune response. Thus, targeting of DCs with beta cell antigens leads to deletion of autoreactive CD8(+) T cells even in the context of ongoing autoimmunity in NOD mice with known tolerance defects. Our results provide support for the development of DC targeting of self antigens for treatment of chronic T cell-mediated autoimmune diseases.
Conflict of interest statement
Conflict of interest statement: R.M.S. advised Celldex, which is developing anti-DEC-205 antibodies for use in humans.
Figures
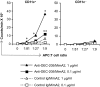
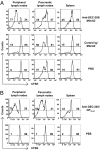
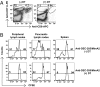


Similar articles
-
DEC-205-mediated antigen targeting to steady-state dendritic cells induces deletion of diabetogenic CD8⁺ T cells independently of PD-1 and PD-L1.Int Immunol. 2013 Nov;25(11):651-60. doi: 10.1093/intimm/dxt031. Epub 2013 Sep 10. Int Immunol. 2013. PMID: 24021877 Free PMC article.
-
Efficient targeting of protein antigen to the dendritic cell receptor DEC-205 in the steady state leads to antigen presentation on major histocompatibility complex class I products and peripheral CD8+ T cell tolerance.J Exp Med. 2002 Dec 16;196(12):1627-38. doi: 10.1084/jem.20021598. J Exp Med. 2002. PMID: 12486105 Free PMC article.
-
CD8+ dendritic cell-mediated tolerance of autoreactive CD4+ T cells is deficient in NOD mice and can be corrected by blocking CD40L.J Leukoc Biol. 2014 Feb;95(2):325-36. doi: 10.1189/jlb.0113013. Epub 2013 Sep 30. J Leukoc Biol. 2014. PMID: 24082013 Free PMC article.
-
Breaking T cell tolerance to beta cell antigens by merocytic dendritic cells.Cell Mol Life Sci. 2011 Sep;68(17):2873-83. doi: 10.1007/s00018-011-0730-6. Epub 2011 May 31. Cell Mol Life Sci. 2011. PMID: 21626409 Free PMC article. Review.
-
Targeted antigen delivery to DEC-205⁺ dendritic cells for tolerogenic vaccination.Rev Diabet Stud. 2012 Winter;9(4):305-18. doi: 10.1900/RDS.2012.9.305. Epub 2012 Dec 28. Rev Diabet Stud. 2012. PMID: 23804268 Free PMC article. Review.
Cited by
-
Cutting-Edge Delivery Systems and Adjuvants in Tolerogenic Vaccines: A Review.Pharmaceutics. 2022 Aug 25;14(9):1782. doi: 10.3390/pharmaceutics14091782. Pharmaceutics. 2022. PMID: 36145531 Free PMC article. Review.
-
T-cell autoantigens in the non-obese diabetic mouse model of autoimmune diabetes.Immunology. 2010 Dec;131(4):459-65. doi: 10.1111/j.1365-2567.2010.03362.x. Epub 2010 Oct 13. Immunology. 2010. PMID: 21039471 Free PMC article. Review.
-
A novel modular antigen delivery system for immuno targeting of human 6-sulfo LacNAc-positive blood dendritic cells (SlanDCs).PLoS One. 2011 Jan 21;6(1):e16315. doi: 10.1371/journal.pone.0016315. PLoS One. 2011. PMID: 21283706 Free PMC article.
-
Autoimmune vitiligo does not require the ongoing priming of naive CD8 T cells for disease progression or associated protection against melanoma.J Immunol. 2014 Feb 15;192(4):1433-9. doi: 10.4049/jimmunol.1302139. Epub 2014 Jan 8. J Immunol. 2014. PMID: 24403535 Free PMC article.
-
Targeting skin dendritic cells to improve intradermal vaccination.Curr Top Microbiol Immunol. 2012;351:113-38. doi: 10.1007/82_2010_118. Curr Top Microbiol Immunol. 2012. PMID: 21253784 Free PMC article. Review.
References
-
- Gianani R, Eisenbarth GS. The stages of type 1A diabetes: 2005. Immunol Rev. 2005;204:232–249. - PubMed
-
- Atkinson MA, Leiter EH. The NOD mouse model of type 1 diabetes: as good as it gets? Nat Med. 1999;5:601–604. - PubMed
-
- DiLorenzo TP, Serreze DV. The good turned ugly: immunopathogenic basis for diabetogenic CD8+ T cells in NOD mice. Immunol Rev. 2005;204:250–263. - PubMed
-
- Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annu Rev Immunol. 2003;21:685–711. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- DK77500/DK/NIDDK NIH HHS/United States
- DK64315/DK/NIDDK NIH HHS/United States
- CA13330/CA/NCI NIH HHS/United States
- AI51573/AI/NIAID NIH HHS/United States
- R01 DK064315/DK/NIDDK NIH HHS/United States
- DK51090/DK/NIDDK NIH HHS/United States
- P30 DK020541/DK/NIDDK NIH HHS/United States
- K01 DK071586/DK/NIDDK NIH HHS/United States
- K99 DK077443/DK/NIDDK NIH HHS/United States
- DK52956/DK/NIDDK NIH HHS/United States
- R29 DK046266/DK/NIDDK NIH HHS/United States
- P60 DK020541/DK/NIDDK NIH HHS/United States
- R01 DK051090/DK/NIDDK NIH HHS/United States
- P30 CA013330/CA/NCI NIH HHS/United States
- DK71586/DK/NIDDK NIH HHS/United States
- R21 DK077500/DK/NIDDK NIH HHS/United States
- DK77443/DK/NIDDK NIH HHS/United States
- R01 DK046266/DK/NIDDK NIH HHS/United States
- R00 DK077443/DK/NIDDK NIH HHS/United States
- P01 DK052956/DK/NIDDK NIH HHS/United States
- DK46266/DK/NIDDK NIH HHS/United States
- P01 AI051573/AI/NIAID NIH HHS/United States
- DK20541/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

