Role of epsin 1 in synaptic vesicle endocytosis
- PMID: 18430801
- PMCID: PMC2359800
- DOI: 10.1073/pnas.0710267105
Role of epsin 1 in synaptic vesicle endocytosis
Abstract
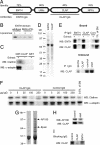
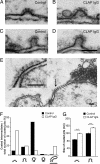
Epsin has been suggested to act as an alternate adaptor in several endocytic pathways. Its role in synaptic vesicle recycling remains, however, unclear. Here, we examined the role of epsin in this process by using the lamprey reticulospinal synapse as a model system. We characterized a lamprey ortholog of epsin 1 and showed that it is accumulated at release sites at rest and also at clathrin-coated pits in the periactive zone during synaptic activity. Disruption of epsin interactions, by presynaptic microinjection of antibodies to either the epsin-N-terminal homology domain (ENTH) or the clathrin/AP2 binding region (CLAP), caused profound loss of vesicles in stimulated synapses. CLAP antibody-injected synapses displayed a massive accumulation of distorted coated structures, including coated vacuoles, whereas in synapses perturbed with ENTH antibodies, very few coated structures were found. In both cases coated pits on the plasma membrane showed a shift to early intermediates (shallow coated pits) and an increase in size. Moreover, in CLAP antibody-injected synapses flat clathrin-coated patches occurred on the plasma membrane. We conclude that epsin is involved in clathrin-mediated synaptic vesicle endocytosis. Our results support a model, based on in vitro studies, suggesting that epsin coordinates curvature generation with coat assembly and further indicating that epsin limits clathrin coat assembly to the size of newly formed vesicles. We propose that these functions of epsin 1 provide an additional mechanism for generation of uniformly sized synaptic vesicles.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Amphiphysin is a component of clathrin coats formed during synaptic vesicle recycling at the lamprey giant synapse.Traffic. 2004 Jul;5(7):514-28. doi: 10.1111/j.1398-9219.2004.00198.x. Traffic. 2004. PMID: 15180828
-
Regulation of synaptic vesicle recycling by complex formation between intersectin 1 and the clathrin adaptor complex AP2.Proc Natl Acad Sci U S A. 2010 Mar 2;107(9):4206-11. doi: 10.1073/pnas.0911073107. Epub 2010 Feb 16. Proc Natl Acad Sci U S A. 2010. PMID: 20160082 Free PMC article.
-
The ENTH and C-terminal domains of Dictyostelium epsin cooperate to regulate the dynamic interaction with clathrin-coated pits.J Cell Sci. 2008 Oct 15;121(Pt 20):3433-44. doi: 10.1242/jcs.032573. Epub 2008 Sep 30. J Cell Sci. 2008. PMID: 18827012
-
Epsin: inducing membrane curvature.Int J Biochem Cell Biol. 2007;39(10):1765-70. doi: 10.1016/j.biocel.2006.12.004. Epub 2007 Jan 17. Int J Biochem Cell Biol. 2007. PMID: 17276129 Review.
-
Giant reticulospinal synapse in lamprey: molecular links between active and periactive zones.Cell Tissue Res. 2006 Nov;326(2):301-10. doi: 10.1007/s00441-006-0216-2. Epub 2006 Jun 20. Cell Tissue Res. 2006. PMID: 16786368 Review.
Cited by
-
Rabex-5 protein regulates the endocytic trafficking pathway of ubiquitinated neural cell adhesion molecule L1.J Biol Chem. 2012 Sep 21;287(39):32312-23. doi: 10.1074/jbc.M112.374322. Epub 2012 Jul 30. J Biol Chem. 2012. PMID: 22846990 Free PMC article.
-
Role of phosphoinositides at the neuronal synapse.Subcell Biochem. 2012;59:131-75. doi: 10.1007/978-94-007-3015-1_5. Subcell Biochem. 2012. PMID: 22374090 Free PMC article. Review.
-
Minimal mesoscale model for protein-mediated vesiculation in clathrin-dependent endocytosis.PLoS Comput Biol. 2010 Sep 9;6(9):e1000926. doi: 10.1371/journal.pcbi.1000926. PLoS Comput Biol. 2010. PMID: 20838575 Free PMC article.
-
Systems biology and physical biology of clathrin-mediated endocytosis.Integr Biol (Camb). 2011 Aug;3(8):803-15. doi: 10.1039/c1ib00036e. Epub 2011 Jul 26. Integr Biol (Camb). 2011. PMID: 21792431 Free PMC article. Review.
-
Embryonic arrest at midgestation and disruption of Notch signaling produced by the absence of both epsin 1 and epsin 2 in mice.Proc Natl Acad Sci U S A. 2009 Aug 18;106(33):13838-43. doi: 10.1073/pnas.0907008106. Epub 2009 Aug 5. Proc Natl Acad Sci U S A. 2009. PMID: 19666558 Free PMC article.
References
-
- Jung N, Haucke V. Clathrin-mediated endocytosis at synapses. Traffic. 2007;8:1129–1136. - PubMed
-
- Ungewickell EJ, Hinrichsen L. Endocytosis: Clathrin-mediated membrane budding. Curr Opin Cell Biol. 2007;19:417–425. - PubMed
-
- Schmid EM, McMahon HT. Integrating molecular and network biology to decode endocytosis. Nature. 2007;448:883–888. - PubMed
-
- Diril MK, Wienisch M, Jung N, Klingauf J, Haucke V. Stonin 2 is an AP-2-dependent endocytic sorting adaptor for synaptotagmin internalization and recycling. Dev Cell. 2006;10:233–244. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

