Treg depletion-enhanced IL-2 treatment facilitates therapy of established tumors using systemically delivered oncolytic virus
- PMID: 18431359
- PMCID: PMC2729455
- DOI: 10.1038/mt.2008.83
Treg depletion-enhanced IL-2 treatment facilitates therapy of established tumors using systemically delivered oncolytic virus
Abstract
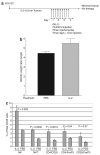
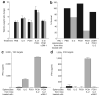
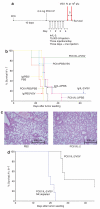
There are several roadblocks that hinder systemic delivery of oncolytic viruses to the sites of metastatic disease. These include the tumor vasculature, which provides a physical barrier to tumor-specific virus extravasation. Although interleukin-2 (IL-2) has been used in antitumor therapy, it is associated with endothelial cell injury, leading to vascular leak syndrome (VLS). Here, we demonstrate that IL-2-mediated VLS, accentuated by depletion of regulatory T cells (Treg), facilitates localization of intravenously (i.v.) delivered oncolytic virus into established tumors in immune-competent mice. IL-2, in association with Treg depletion, generates "hyperactivated" natural killer (NK) cells, possessing antitumor activity and secreting factors that facilitate virus spread/replication throughout the tumor by disrupting the tumor architecture. As a result, the combination of Treg depletion/IL-2 and systemic oncolytic virotherapy was found to be significantly more therapeutic against established disease than either treatment alone. These data demonstrate that it is possible to combine biological therapy with oncolytic virotherapy to generate systemic therapy against established tumors.
Figures



Similar articles
-
Treg Depletion-enhanced IL-2 Treatment Facilitates Therapy of Established Tumors Using Systemically Delivered Oncolytic Virus.Mol Ther. 2008 Jul;16(7):1217-1226. doi: 10.1038/mt.2008.83. Epub 2016 Dec 8. Mol Ther. 2008. PMID: 28178481
-
Improved systemic delivery of oncolytic reovirus to established tumors using preconditioning with cyclophosphamide-mediated Treg modulation and interleukin-2.Clin Cancer Res. 2009 Jan 15;15(2):561-9. doi: 10.1158/1078-0432.CCR-08-1688. Clin Cancer Res. 2009. PMID: 19147761 Free PMC article.
-
Use of biological therapy to enhance both virotherapy and adoptive T-cell therapy for cancer.Mol Ther. 2008 Dec;16(12):1910-8. doi: 10.1038/mt.2008.212. Epub 2008 Sep 30. Mol Ther. 2008. PMID: 18827807 Free PMC article.
-
Mounting a strategic offense: fighting tumor vasculature with oncolytic viruses.Trends Mol Med. 2013 Jun;19(6):378-92. doi: 10.1016/j.molmed.2013.02.008. Epub 2013 Mar 27. Trends Mol Med. 2013. PMID: 23540715 Review.
-
Oncolytic viruses in the treatment of cancer: a review of current strategies.Pathol Oncol Res. 2012 Oct;18(4):771-81. doi: 10.1007/s12253-012-9548-2. Epub 2012 Jun 20. Pathol Oncol Res. 2012. PMID: 22714538 Review.
Cited by
-
Interaction between natural killer cells and regulatory T cells: perspectives for immunotherapy.Cell Mol Immunol. 2013 May;10(3):222-9. doi: 10.1038/cmi.2013.2. Epub 2013 Mar 25. Cell Mol Immunol. 2013. PMID: 23524654 Free PMC article. Review.
-
CD4+CD25+ Cells Are Essential for Maintaining Immune Tolerance in Chickens Inoculated with Bovine Serum Albumin at the Late Stage of Embryonic Development.Vet Sci. 2020 Oct 3;7(4):150. doi: 10.3390/vetsci7040150. Vet Sci. 2020. PMID: 33022909 Free PMC article.
-
Oncolytic vesicular stomatitis virus in an immunocompetent model of MUC1-positive or MUC1-null pancreatic ductal adenocarcinoma.J Virol. 2013 Sep;87(18):10283-94. doi: 10.1128/JVI.01412-13. Epub 2013 Jul 17. J Virol. 2013. PMID: 23864625 Free PMC article.
-
Vesicular stomatitis virus as a flexible platform for oncolytic virotherapy against cancer.J Gen Virol. 2012 Dec;93(Pt 12):2529-2545. doi: 10.1099/vir.0.046672-0. Epub 2012 Oct 10. J Gen Virol. 2012. PMID: 23052398 Free PMC article. Review.
-
Enhancement of shrimp antiviral immune response through caspase-dependent apoptosis by small molecules.Mar Biotechnol (NY). 2011 Jun;13(3):575-83. doi: 10.1007/s10126-010-9328-5. Epub 2010 Oct 9. Mar Biotechnol (NY). 2011. PMID: 20936319
References
-
- Fisher K. Striking out at disseminated metastases: the systemic delivery of oncolytic viruses. Curr Opin Mol Ther. 2006;8:301–313. - PubMed
-
- Harrington K, Alvarez-Vallina L, Crittenden M, Gough M, Chong H, Diaz RM, et al. Cells as vehicles for cancer gene therapy: the missing link between targeted vectors and systemic delivery? Hum Gene Ther. 2002;13:1263–1280. - PubMed
-
- Friedman A, Tian JP, Fulci G, Chiocca EA, Wang J. Glioma virotherapy: effects of innate immune suppression and increased viral replication capacity. Cancer Res. 2006;66:2314–2319. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

