Hypospadias and anorectal malformations mediated by Eph/ephrin signaling
- PMID: 18431460
- PMCID: PMC2329588
- DOI: 10.1016/j.jpurol.2007.01.199
Hypospadias and anorectal malformations mediated by Eph/ephrin signaling
Abstract
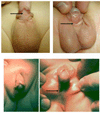
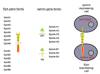
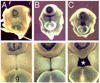
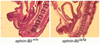
PURPOSE: Despite extensive research, the molecular basis of hypospadias and anorectal malformations is poorly understood, likely due to a multifactorial basis. The incidence of hypospadias is increasing, thus making research in this area warranted and timely. This review presents recent molecular work broadening our understanding of these disorders. MATERIALS AND METHODS: A brief review of our recent work and the literature on the role of Eph/ephrin signaling in hypospadias and anorectal malformations is presented. RESULTS: Genetically engineered mice mutant for ephrin-B2 or EphB2;EphB3 manifest a variety of genitourinary and anorectal malformations. Approximately 40% of adult male heterozygous mice demonstrate perineal hypospadias. Although homozygous mice die soon after birth, 100% of homozygous males demonstrate high imperforate anus with urethral anomalies and 100% of homozygous females demonstrate persistent cloaca. Male mice compound homozygous for EphB2(ki/ki);EphB3(Delta/Delta)/ also demonstrate hypospadias. CONCLUSIONS: These mouse models provide compelling evidence of the role of B-class Eph/ephrin signaling in genitourinary/anorectal development and add to our mechanistic and molecular understanding of normal and abnormal embryonic development. As research on the B-class Ephs and ephrins continues, they will likely be shown to be molecular contributors to the multifactorial basis of hypospadias and anorectal malformations in humans as well.
Figures






References
-
- Paulozzi LJ, Erickson D, Jackson RJ. Hypospadias trends in two US surveillance systems. Pediatrics. 1997;100:831–834. - PubMed
-
- Wilson JD, Griffin JE, George FW, Leshin M. The endocrine control of male phenotypic development. Aust J Biol Sci. 1983;36:101–128. - PubMed
-
- Wilson JD, Griffin JE, Russell DW. Steroid 5 alpha-reductase 2 deficiency. Endocr Rev. 1993;14:577. - PubMed
-
- Holmes N, Miller W, Baskin L. Lack of defects in androgen production in children with hypospadias. J Clin Endocrinol Metab. 2004;89:2811–2816. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

