Sorting signals, N-terminal modifications and abundance of the chloroplast proteome
- PMID: 18431481
- PMCID: PMC2291561
- DOI: 10.1371/journal.pone.0001994
Sorting signals, N-terminal modifications and abundance of the chloroplast proteome
Abstract
Characterization of the chloroplast proteome is needed to understand the essential contribution of the chloroplast to plant growth and development. Here we present a large scale analysis by nanoLC-Q-TOF and nanoLC-LTQ-Orbitrap mass spectrometry (MS) of ten independent chloroplast preparations from Arabidopsis thaliana which unambiguously identified 1325 proteins. Novel proteins include various kinases and putative nucleotide binding proteins. Based on repeated and independent MS based protein identifications requiring multiple matched peptide sequences, as well as literature, 916 nuclear-encoded proteins were assigned with high confidence to the plastid, of which 86% had a predicted chloroplast transit peptide (cTP). The protein abundance of soluble stromal proteins was calculated from normalized spectral counts from LTQ-Obitrap analysis and was found to cover four orders of magnitude. Comparison to gel-based quantification demonstrates that 'spectral counting' can provide large scale protein quantification for Arabidopsis. This quantitative information was used to determine possible biases for protein targeting prediction by TargetP and also to understand the significance of protein contaminants. The abundance data for 550 stromal proteins was used to understand abundance of metabolic pathways and chloroplast processes. We highlight the abundance of 48 stromal proteins involved in post-translational proteome homeostasis (including aminopeptidases, proteases, deformylases, chaperones, protein sorting components) and discuss the biological implications. N-terminal modifications were identified for a subset of nuclear- and chloroplast-encoded proteins and a novel N-terminal acetylation motif was discovered. Analysis of cTPs and their cleavage sites of Arabidopsis chloroplast proteins, as well as their predicted rice homologues, identified new species-dependent features, which will facilitate improved subcellular localization prediction. No evidence was found for suggested targeting via the secretory system. This study provides the most comprehensive chloroplast proteome analysis to date and an expanded Plant Proteome Database (PPDB) in which all MS data are projected on identified gene models.
Conflict of interest statement
Figures
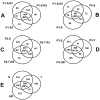
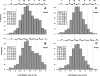
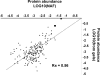


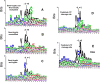

References
-
- von Heijne G, Steppuhn J, Hermann SG. Domain structure of mitochondrial and chloroplast targeting peptides. Eur J Biochemistry. 1989;80:535–545. - PubMed
-
- Emanuelsson O, Brunak S, von Heijne G, Nielsen H. Locating proteins in the cell using TargetP, SignalP and related tools. Nat Protoc. 2007;2(4):953–971. - PubMed
-
- Richly E, Leister D. An improved prediction of chloroplast proteins reveals diversities and commonalities in the chloroplast proteomes of Arabidopsis and rice. Gene. 2004;329:11–16. - PubMed
-
- van Wijk KJ. Plastid proteomics. Plant Physiol Biochem. 2004;42(12):963–77. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

