Metabolic and immune activation effects of treatment interruption in chronic HIV-1 infection: implications for cardiovascular risk
- PMID: 18431498
- PMCID: PMC2292263
- DOI: 10.1371/journal.pone.0002021
Metabolic and immune activation effects of treatment interruption in chronic HIV-1 infection: implications for cardiovascular risk
Abstract
Background: Concern about costs and antiretroviral therapy (ART)-associated toxicities led to the consideration of CD4 driven strategies for the management of HIV. That approach was evaluated in the SMART trial that reported an unexpected increase of cardiovascular events after treatment interruption (TI). Our goal was to evaluate fasting metabolic changes associated with interruption of antiretroviral therapy and relate them to changes of immune activation markers and cardiovascular risk.
Methodology: ACTG 5102 enrolled 47 HIV-1-infected subjects on stable ART, with <200 HIV RNA copies/mL and CD4 cell count >or=500 cells/microL. Subjects were randomly assigned to continue ART for 18 weeks with or without 3 cycles of interleukin-2 (IL-2) (cycle = 4.5 million IU sc BID x 5 days every 8 weeks). After 18 weeks ART was discontinued in all subjects until the CD4 cell count dropped below 350 cells/microL. Glucose and lipid parameters were evaluated every 8 weeks initially and at weeks 2, 4, 8 and every 8 weeks after TI. Immune activation was evaluated by flow-cytometry and soluble TNFR2 levels.
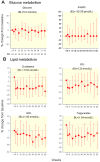
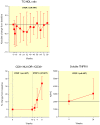
Principal findings: By week 8 of TI, levels of total cholesterol (TC) (median (Q1, Q3) (-0.73 (-1.19, -0.18) mmol/L, p<0.0001), LDL, HDL cholesterol (-0.36(-0.73,-0.03)mmol/L, p = 0.0007 and -0.05(-0.26,0.03), p = 0.0033, respectively) and triglycerides decreased (-0.40 (-0.84, 0.07) mmol/L, p = 0.005). However the TC/HDL ratio remained unchanged (-0.09 (-1.2, 0.5), p = 0.2). Glucose and insulin levels did not change (p = 0.6 and 0.8, respectively). After TI there was marked increase in immune activation (CD8+/HLA-DR+/CD38+ cells, 34% (13, 43), p<0.0001) and soluble TNFR2 (1089 ng/L (-189, 1655), p = 0.0008) coinciding with the rebound of HIV viremia.
Conclusions: Our data suggests that interrupting antiretroviral therapy does not reduce cardiovascular disease (CVD) risk, as the improvements in lipid parameters are modest and overshadowed by the decreased HDL levels. Increased immune cell activation and systemic inflammatory responses associated with recrudescent HIV viremia may provide a more cogent explanation for the increased cardiovascular risk associated with treatment interruption and HIV infection.
Trial registration: ClinicalTrials.gov NCT00015704.
Conflict of interest statement
Figures

References
-
- Grinspoon S, Carr A. Cardiovascular risk and body-fat abnormalities in HIV-infected adults. N Engl J Med. 2005;352:48–62. - PubMed
-
- Friis-Moller N, Sabin CA, Weber R, d'Arminio Monforte A, El-Sadr WM, et al. Combination antiretroviral therapy and the risk of myocardial infarction. N Engl J Med. 2003;349:1993–2003. - PubMed
-
- Friis-Moller N, Reiss P, Sabin CA, Weber R, Monforte A, et al. Class of antiretroviral drugs and the risk of myocardial infarction. N Engl J Med. 2007;356:1723–1735. - PubMed
-
- Dybul M, Nies-Kraske E, Daucher M, Hertogs K, Hallahan CW, et al. Long-cycle structured intermittent versus continuous highly active antiretroviral therapy for the treatment of chronic infection with human immunodeficiency virus: effects on drug toxicity and on immunologic and virologic parameters. J Infect Dis. 2003;188:388–396. - PubMed
-
- Dybul M, Nies-Kraske E, Dewar R, Maldarelli F, Hallahan CW, et al. A proof-of-concept study of short-cycle intermittent antiretroviral therapy with a once-daily regimen of didanosine, lamivudine, and efavirenz for the treatment of chronic HIV infection. J Infect Dis. 2004;189:1974–1982. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- AI25879/AI/NIAID NIH HHS/United States
- AI25868/AI/NIAID NIH HHS/United States
- U01 AI039156/AI/NIAID NIH HHS/United States
- AI27666/AI/NIAID NIH HHS/United States
- AI27661/AI/NIAID NIH HHS/United States
- M01 RR000070/RR/NCRR NIH HHS/United States
- U01 AI025879/AI/NIAID NIH HHS/United States
- AI46370/AI/NIAID NIH HHS/United States
- U01 AI027661/AI/NIAID NIH HHS/United States
- U01 AI025903/AI/NIAID NIH HHS/United States
- U01AI38858/AI/NIAID NIH HHS/United States
- U01 AI038858/AI/NIAID NIH HHS/United States
- U01 AI046370/AI/NIAID NIH HHS/United States
- U01 AI027666/AI/NIAID NIH HHS/United States
- AI39156/AI/NIAID NIH HHS/United States
- AI25903/AI/NIAID NIH HHS/United States
- U01 AI025868/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

