STAT3 and STAT1 mediate IL-11-dependent and inflammation-associated gastric tumorigenesis in gp130 receptor mutant mice
- PMID: 18431520
- PMCID: PMC2323192
- DOI: 10.1172/JCI34944
STAT3 and STAT1 mediate IL-11-dependent and inflammation-associated gastric tumorigenesis in gp130 receptor mutant mice
Abstract
Deregulated activation of STAT3 is frequently associated with many human hematological and epithelial malignancies, including gastric cancer. While exaggerated STAT3 signaling facilitates an antiapoptotic, proangiogenic, and proproliferative environment for neoplastic cells, the molecular mechanisms leading to STAT3 hyperactivation remain poorly understood. Using the gp130(Y757F/Y757F) mouse model of gastric cancer, which carries a mutated gp130 cytokine receptor signaling subunit that cannot bind the negative regulator of cytokine signaling SOCS3 and is characterized by hyperactivation of the signaling molecules STAT1 and STAT3, we have provided genetic evidence that IL-11 promotes chronic gastric inflammation and associated tumorigenesis. Expression of IL-11 was increased in gastric tumors in gp130(Y757F/Y757F) mice, when compared with unaffected gastric tissue in wild-type mice, while gp130(Y757F/Y757F) mice lacking the IL-11 ligand-binding receptor subunit (IL-11Ralpha) showed normal gastric STAT3 activation and IL-11 expression and failed to develop gastric tumors. Furthermore, reducing STAT3 activity in gp130(Y757F/Y757F) mice, either genetically or by therapeutic administration of STAT3 antisense oligonucleotides, normalized gastric IL-11 expression and alleviated gastric tumor burden. Surprisingly, the genetic reduction of STAT1 expression also reduced gastric tumorigenesis in gp130(Y757F/Y757F) mice and coincided with reduced gastric inflammation and IL-11 expression. Collectively, our data have identified IL-11 as a crucial cytokine promoting chronic gastric inflammation and associated tumorigenesis mediated by excessive activation of STAT3 and STAT1.
Figures

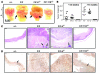

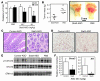

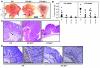


Comment in
-
What lurks beneath: IL-11, via Stat3, promotes inflammation-associated gastric tumorigenesis.J Clin Invest. 2008 May;118(5):1628-31. doi: 10.1172/JCI35344. J Clin Invest. 2008. PMID: 18431518 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

