Physiology and pathophysiology of the vasopressin-regulated renal water reabsorption
- PMID: 18431594
- PMCID: PMC2518081
- DOI: 10.1007/s00424-008-0498-1
Physiology and pathophysiology of the vasopressin-regulated renal water reabsorption
Abstract
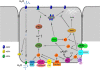
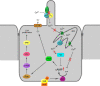
To prevent dehydration, terrestrial animals and humans have developed a sensitive and versatile system to maintain their water homeostasis. In states of hypernatremia or hypovolemia, the antidiuretic hormone vasopressin (AVP) is released from the pituitary and binds its type-2 receptor in renal principal cells. This triggers an intracellular cAMP signaling cascade, which phosphorylates aquaporin-2 (AQP2) and targets the channel to the apical plasma membrane. Driven by an osmotic gradient, pro-urinary water then passes the membrane through AQP2 and leaves the cell on the basolateral side via AQP3 and AQP4 water channels. When water homeostasis is restored, AVP levels decline, and AQP2 is internalized from the plasma membrane, leaving the plasma membrane watertight again. The action of AVP is counterbalanced by several hormones like prostaglandin E2, bradykinin, dopamine, endothelin-1, acetylcholine, epidermal growth factor, and purines. Moreover, AQP2 is strongly involved in the pathophysiology of disorders characterized by renal concentrating defects, as well as conditions associated with severe water retention. This review focuses on our recent increase in understanding of the molecular mechanisms underlying AVP-regulated renal water transport in both health and disease.
Figures

References
-
- Ahrabi AK, Terryn S, Valenti G, Caron N, Serradeil-Le GC, Raufaste D, Nielsen S, Horie S, Verbavatz JM, Devuyst O. PKD1 haploinsufficiency causes a syndrome of inappropriate antidiuresis in mice. J Am Soc Nephrol. 2007;18:1740–1753. - PubMed
-
- Alfie ME, Alim S, Mehta D, Shesely EG, Carretero OA. An enhanced effect of arginine vasopressin in bradykinin B2 receptor null mutant mice. Hypertension. 1999;33:1436–1440. - PubMed
-
- Ando Y, Asano Y. Functional evidence for an apical V1 receptor in rabbit cortical. Am J Physiol. 1993;264:F467–F471. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

