NF-kappaB links innate immunity to the hypoxic response through transcriptional regulation of HIF-1alpha
- PMID: 18432192
- PMCID: PMC2669289
- DOI: 10.1038/nature06905
NF-kappaB links innate immunity to the hypoxic response through transcriptional regulation of HIF-1alpha
Abstract
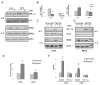
The hypoxic response is an ancient stress response triggered by low ambient oxygen (O2) (ref. 1) and controlled by hypoxia-inducible transcription factor-1 (HIF-1), whose alpha subunit is rapidly degraded under normoxia but stabilized when O2-dependent prolyl hydroxylases (PHDs) that target its O2-dependent degradation domain are inhibited. Thus, the amount of HIF-1alpha, which controls genes involved in energy metabolism and angiogenesis, is regulated post-translationally. Another ancient stress response is the innate immune response, regulated by several transcription factors, among which NF-kappaB plays a central role. NF-kappaB activation is controlled by IkappaB kinases (IKK), mainly IKK-beta, needed for phosphorylation-induced degradation of IkappaB inhibitors in response to infection and inflammation. IKK-beta is modestly activated in hypoxic cell cultures when PHDs that attenuate its activation are inhibited. However, defining the relationship between NF-kappaB and HIF-1alpha has proven elusive. Using in vitro systems, it was reported that HIF-1alpha activates NF-kappaB, that NF-kappaB controls HIF-1alpha transcription and that HIF-1alpha activation may be concurrent with inhibition of NF-kappaB. Here we show, with the use of mice lacking IKK-beta in different cell types, that NF-kappaB is a critical transcriptional activator of HIF-1alpha and that basal NF-kappaB activity is required for HIF-1alpha protein accumulation under hypoxia in cultured cells and in the liver and brain of hypoxic animals. IKK-beta deficiency results in defective induction of HIF-1alpha target genes including vascular endothelial growth factor. IKK-beta is also essential for HIF-1alpha accumulation in macrophages experiencing a bacterial infection. Hence, IKK-beta is an important physiological contributor to the hypoxic response, linking it to innate immunity and inflammation.
Figures

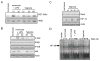


References
-
- Maxwell PH, MSW, GWC, SCC, ECV, MEC, Wykoff CC, Pugh CW, Maher ER, Ratcliffe PJ. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature. 1999;399:271–275. - PubMed
-
- Semenza G. HIF-1, O(2), and the 3 PHDs: how animal cells signal hypoxia to the nucleu. Cell. 2001;107:1–3. - PubMed
-
- Schofield CJ, Ratcliffe PJ. Oxygen sensing by HIF hydroxylase. Nat Rev Mol Cell Biol. 2004;5:343–354. - PubMed
-
- Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

