Three-dimensional random access multiphoton microscopy for functional imaging of neuronal activity
- PMID: 18432198
- PMCID: PMC2747788
- DOI: 10.1038/nn.2116
Three-dimensional random access multiphoton microscopy for functional imaging of neuronal activity
Abstract
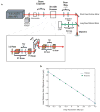
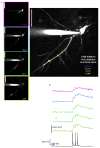
The dynamic ability of neuronal dendrites to shape and integrate synaptic responses is the hallmark of information processing in the brain. Effectively studying this phenomenon requires concurrent measurements at multiple sites on live neurons. Substantial progress has been made by optical imaging systems that combine confocal and multiphoton microscopy with inertia-free laser scanning. However, all of the systems developed so far restrict fast imaging to two dimensions. This severely limits the extent to which neurons can be studied, as they represent complex three-dimensional structures. Here we present a new imaging system that utilizes a unique arrangement of acousto-optic deflectors to steer a focused, ultra-fast laser beam to arbitrary locations in three-dimensional space without moving the objective lens. As we demonstrate, this highly versatile random-access multiphoton microscope supports functional imaging of complex three-dimensional cellular structures such as neuronal dendrites or neural populations at acquisition rates on the order of tens of kilohertz.
Figures




References
-
- Megias M, Emri Z, Freund TF, Gulyas AI. Total number and distribution of inhibitory and excitatory synapses on hippocampal CA1 pyramidal cells. Neuroscience. 2001;102:527–540. - PubMed
-
- Ambros-Ingerson J, Holmes WR. Analysis and comparison of morphological reconstructions of hippocampal field CA1 pyramidal cells. Hippocampus. 2005;15:302–315. - PubMed
-
- Losonczy A, Magee JC. Integrative properties of radial oblique dendrites in hippocampal CA1 pyramidal neurons. Neuron. 2006;50:291–307. - PubMed
-
- Helmchen F, Svoboda K, Denk W, Tank DW. In vivo dendritic calcium dynamics in deep-layer cortical pyramidal neurons. Nat Neurosci. 1999;2:989–996. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

