Evolution of a new enzyme activity from the same motif fold
- PMID: 18433454
- PMCID: PMC2574927
- DOI: 10.1111/j.1365-2958.2008.06241.x
Evolution of a new enzyme activity from the same motif fold
Abstract
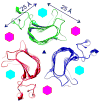
The host cell recognition protein of the Escherichia coli bacteriophage HK620 is a large homotrimeric tailspike that cleaves the O18A1 type O antigen. The crystal structure of HK620 tailspike determined in the apo and substrate-bound form is reported by Barbirz et al. in this issue of Molecular Microbiology. Lacking detectable sequence similarity, the fold and overall organization of the HK620 tailspike are similar to those of the tailspikes of the related phages P22 and Sf6. The substrate-binding site is intrasubunit in P22 and HK620 tailspikes, but intersubunit in Sf6, demonstrating how phages can adapt the same protein fold to recognize different substrates.
Figures
Comment on
-
Crystal structure of Escherichia coli phage HK620 tailspike: podoviral tailspike endoglycosidase modules are evolutionarily related.Mol Microbiol. 2008 Jul;69(2):303-16. doi: 10.1111/j.1365-2958.2008.06311.x. Mol Microbiol. 2008. PMID: 18547389
References
-
- Barbirz S, Müller JJ, Utrecht C, Clark AJ, Heinemann U, Seckler R. Crystal structure of E. coli phage HK620 tailspike reveals that podoviral tailspike endoglycosidase modules are evolutionary related. Molecular Microbiology. 2008 this issue. - PubMed
-
- Casjens S, Winn-Stapley DA, Gilcrease EB, Morona R, Kuhlewein C, Chua JE, Manning PA, Inwood W, Clark AJ. The chromosome of Shigella flexneri bacteriophage Sf6: complete nucleotide sequence, genetic mosaicism, and DNA packaging. J Mol Biol. 2004;339:379–394. - PubMed
-
- Lander GC, Tang L, Casjens SR, Gilcrease EB, Prevelige P, Poliakov A, Potter CS, Carragher B, Johnson JE. The structure of an infectious P22 virion shows the signal for headful DNA packaging. Science. 2006;312:1791–1795. - PubMed
-
- Leiman PG, Battisti AJ, Bowman VD, Stummeyer K, Muhlenhoff M, Gerardy-Schahn R, Scholl D, Molineux IJ. The structures of bacteriophages K1E and K1–5 explain processive degradation of polysaccharide capsules and evolution of new host specificities. J Mol Biol. 2007;371:836–849. - PubMed
-
- Müller JJ, Barbirz S, Heinle K, Freiberg A, Seckler R, Heinemann U. An inter-subunit active site between supercoiled parallel beta-helices in the trimeric tailspike endorhamnosidase of Shigella flexneri phage Sf6. Structure. 2008 in the press. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

