Molecular weight dependence of the depletion attraction and its effects on the competitive adsorption of lung surfactant
- PMID: 18433716
- PMCID: PMC2575753
- DOI: 10.1016/j.bbamem.2008.03.019
Molecular weight dependence of the depletion attraction and its effects on the competitive adsorption of lung surfactant
Abstract
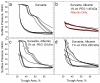
Albumin competes with lung surfactant for the air-water interface, resulting in decreased surfactant adsorption and increased surface tension. Polyethylene glycol (PEG) and other hydrophilic polymers restore the normal rate of surfactant adsorption to the interface, which re-establishes low surface tensions on compression. PEG does so by generating an entropic depletion attraction between the surfactant aggregates and interface, reducing the energy barrier to adsorption imposed by the albumin. For a fixed composition of 10 g/L (1% wt.), surfactant adsorption increases with the 0.1 power of PEG molecular weight from 6 kDa-35 kDa as predicted by simple excluded volume models of the depletion attraction. The range of the depletion attraction for PEG with a molecular weight below 6 kDa is less than the dimensions of albumin and there is no effect on surfactant adsorption. PEG greater than 35 kDa reaches the overlap concentration at 1% wt. resulting in both decreased depletion attraction and decreased surfactant adsorption. Fluorescence images reveal that the depletion attraction causes the surfactant to break through the albumin film at the air-water interface to spread as a monolayer. During this transition, there is a coexistence of immiscible albumin and surfactant domains. Surface pressures well above the normal equilibrium surface pressure of albumin are necessary to force the albumin from the interface during film compression.
Figures


References
-
- Clements JA, Avery ME. American Journal of Respiratory and Critical Care Medicine. 1998;157:S59–S66. - PubMed
-
- Notter R. Lung surfactant: basic science and clinical applications. Vol. 149. Marcel Dekker; New York: 2000.
-
- Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, Neff M, Stern EJ, Hudson LD. New England Journal of Medicine. 2005;353:1685–1693. - PubMed
-
- Kesecioglu J, Haitsma JJ. Current Opinion in Critical Care. 2006;12:55–60. - PubMed
-
- Tashiro K, Cui XG, Kobayashi T, Curstedt T, Robertson B. Biology of the Neonate. 2003;83:49–56. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

