Comparing surface-based and volume-based analyses of functional neuroimaging data in patients with schizophrenia
- PMID: 18434199
- PMCID: PMC2527864
- DOI: 10.1016/j.neuroimage.2008.02.052
Comparing surface-based and volume-based analyses of functional neuroimaging data in patients with schizophrenia
Abstract
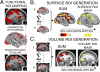
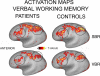
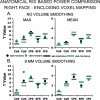
A major challenge in functional neuroimaging is to cope with individual variability in cortical structure and function. Most analyses of cortical function compensate for variability using affine or low-dimensional nonlinear volume-based registration (VBR) of individual subjects to an atlas, which does not explicitly take into account the geometry of cortical convolutions. A promising alternative is to use surface-based registration (SBR), which capitalizes on explicit surface representations of cortical folding patterns in individual subjects. In this study, we directly compare results from SBR and affine VBR in a study of working memory in healthy controls and patients with schizophrenia (SCZ). Each subject's structural scan was used for cortical surface reconstruction using the SureFit method. fMRI data were mapped directly onto individual cortical surface models, and each hemisphere was registered to the population-average PALS-B12 atlas using landmark-constrained SBR. The precision with which cortical sulci were aligned was much greater for SBR than VBR. SBR produced superior alignment precision across the entire cortex, and this benefit was greater in patients with schizophrenia. We demonstrate that spatial smoothing on the surface provides better resolution and signal preservation than a comparable degree of smoothing in the volume domain. Lastly, the statistical power of functional activation in the working memory task was greater for SBR than for VBR. These results indicate that SBR provides significant advantages over affine VBR when analyzing cortical fMRI activations. Furthermore, these improvements can be even greater in disorders that have associated structural abnormalities.
Figures








References
-
- Acton PD, Friston KJ. Statistical parametric mapping in functional neuroimaging: beyond PET and fMRI activation studies. Eur. J. Nucl. Med. 1998;25:663–667. - PubMed
-
- Ashburner J, K.J. Friston KJ. High-Dimensional Nonlinear Image Registration. NeuroImage. 1998;7(4):S737. 1998. - PubMed
-
- Baddeley AD. Working memory. Oxford University Press; New York: 1986.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

