Asymmetrical stimulus generalization following differential fear conditioning
- PMID: 18434217
- PMCID: PMC2516404
- DOI: 10.1016/j.nlm.2008.02.009
Asymmetrical stimulus generalization following differential fear conditioning
Erratum in
- Neurobiol Learn Mem. 2008 Oct;90(3):588
Abstract
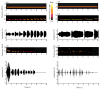
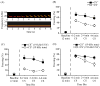
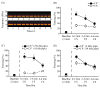
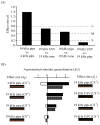
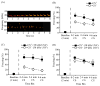
Rodent ultrasonic vocalizations (USVs) are ethologically critical social signals. Rats emit 22kHz USVs and 50kHz USVs, respectively, in conjunction with negative and positive affective states. Little is known about what controls emotional reactivity to these social signals. Using male Sprague-Dawley rats, we examined unconditional and conditional freezing behavior in response to the following auditory stimuli: three 22kHz USVs, a discontinuous tone whose frequency and on-off pattern matched one of the USVs, a continuous tone with the same or lower frequencies, a 4kHz discontinuous tone with an on-off pattern matched to one of the USVs, and a 50kHz USV. There were no differences among these stimuli in terms of the unconditional elicitation of freezing behavior. Thus, the stimuli were equally neutral before conditioning. During differential fear conditioning, one of these stimuli (the CS(+)) always co-terminated with a footshock unconditional stimulus (US) and another stimulus (the CS(-)) was explicitly unpaired with the US. There were no significant differences among these cues in CS(+)-elicited freezing behavior. Thus, the stimuli were equally salient or effective as cues in supporting fear conditioning. When the CS(+) was a 22kHz USV or a similar stimulus, rats discriminated based on the principal frequency and/or the temporal pattern of the stimulus. However, when these same stimuli served as the CS(-), discrimination failed due to generalization from the CS(+). Thus, the stimuli differed markedly in the specificity of conditioning. This strikingly asymmetrical stimulus generalization is a novel bias in discrimination.
Figures
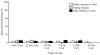



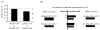

References
-
- Anderson JW. The production of ultrasonic sounds by laboratory rats and other mammals. Science. 1954;119(3101):808–809. - PubMed
-
- Barfield RJ, Geyer LA. Sexual behavior: Ultrasonic postejaculatory song of the male rat. Science. 1972;176(41):1349–1350. - PubMed
-
- Bhagavan S, Smith BH. Olfactory conditioning in the honey bee, Apis mellifera: Effects of odor intensity. Physiology & Behavior. 1997;61(1):107–117. - PubMed
-
- Blair HT, Sotres-Bayon F, Moita MA, LeDoux JE. The lateral amygdala processes the value of conditioned and unconditioned aversive stimuli. Neuroscience. 2005;133(2):561–569. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

