Abundance and stability of complexes containing inactive G protein-coupled receptors and G proteins
- PMID: 18434433
- PMCID: PMC2493464
- DOI: 10.1096/fj.08-105775
Abundance and stability of complexes containing inactive G protein-coupled receptors and G proteins
Abstract
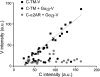
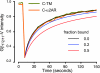
G protein-coupled receptors (GPCRs) interact directly with heterotrimeric G proteins to transduce physiological signals. Early studies of this interaction concluded that GPCRs (R) and G proteins (G) collide with each other randomly after receptor activation and that R-G complexes are transient. More recent studies have suggested that inactive R and G are preassembled (precoupled) as stable R-G complexes. Here we examine the stability of complexes formed between cyan fluorescent protein-labeled alpha(2A)-adrenoreceptors (C-alpha2ARs) and G proteins in cells using fluorescence recovery after photobleaching (FRAP). Labeled G proteins diffused in the plasma membrane with equal mobility in the absence and presence of immobile C-alpha2ARs. Immobile C-alpha2ARs activated labeled G proteins, demonstrating functional coupling without stable physical association. In contrast, a stable R-G interaction was detected when G proteins were deprived of nucleotides and C-alpha2ARs were active, as predicted by the ternary complex model. Overexpression of regulator of G protein signaling 4 (RGS4) accelerated the onset of effector activation but did not detectably alter the interaction between C-alpha2ARs and G proteins. We conclude that at most a small fraction of C-alpha2ARs and G proteins exist as R-G complexes at any moment.
Figures

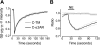
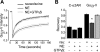

References
-
- Tolkovsky A M, Levitzki A. Mode of coupling between the beta-adrenergic receptor and adenylate cyclase in turkey erythrocytes. Biochemistry. 1978;17:3795. - PubMed
-
- Pugh E N, Jr, Lamb T D. Amplification and kinetics of the activation steps in phototransduction. Biochim Biophys Acta. 1993;1141:111–149. - PubMed
-
- Gales C, Van Durm J J, Schaak S, Pontier S, Percherancier Y, Audet M, Paris H, Bouvier M. Probing the activation-promoted structural rearrangements in preassembled receptor-G protein complexes. Nat Struct Mol Biol. 2006;13:778–786. - PubMed
-
- Neubig R R, Gantzos R D, Thomsen W J. Mechanism of agonist and antagonist binding to alpha 2 adrenergic receptors: evidence for a precoupled receptor-guanine nucleotide protein complex. Biochemistry. 1988;27:2374–2384. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

