n-Alcohols inhibit voltage-gated Na+ channels expressed in Xenopus oocytes
- PMID: 18434586
- PMCID: PMC2575017
- DOI: 10.1124/jpet.108.138370
n-Alcohols inhibit voltage-gated Na+ channels expressed in Xenopus oocytes
Abstract
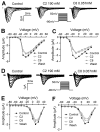
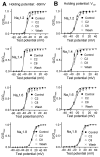
Voltage-gated sodium channels are essential for the initiation and propagation of action potentials in excitable cells and are known as a target of local anesthetics. In addition, inhibition of sodium channels by volatile anesthetics has been proposed as a mechanism of general anesthesia. The n-alcohols produce anesthesia, and their potency increases with carbon number until a "cut-off" is reached. In this study, we examined effects of a range of n-alcohols on Na(v)1.2 subunits to determine the alcohol cut-off for this channel. We also studied the effect of a short-chain alcohol (ethanol) and a long-chain alcohol (octanol) on Na(v)1.2, Na(v)1.4, Na(v)1.6, and Na(v)1.8 subunits, and we investigated the effects of alcohol on channel kinetics. Ethanol and octanol inhibited sodium currents of all subunits, and the inhibition of the Na(v)1.2 channel by n-alcohols indicated a cut-off at nonanol. Ethanol and octanol produced open-channel block, which was more pronounced for Na(v)1.8 than for the other sodium channels. Inhibition of Na(v)1.2 was due to decreased activation and increased inactivation. These results suggest that sodium channels may have a hydrophobic binding site for n-alcohols and demonstrate the differences in the kinetic mechanisms of inhibition for n-alcohols and inhaled anesthetics.
Figures






Similar articles
-
Effects of alcohols and anesthetics on recombinant voltage-gated Na+ channels.J Pharmacol Exp Ther. 2004 Jun;309(3):987-94. doi: 10.1124/jpet.103.064063. Epub 2004 Feb 20. J Pharmacol Exp Ther. 2004. PMID: 14978193
-
The effects of volatile aromatic anesthetics on voltage-gated Na+ channels expressed in Xenopus oocytes.Anesth Analg. 2008 Nov;107(5):1579-86. doi: 10.1213/ane.0b013e318184b966. Anesth Analg. 2008. PMID: 18931215 Free PMC article.
-
Inhibition of voltage-gated Na(+) currents in sensory neurones by the sea anemone toxin APETx2.Br J Pharmacol. 2012 Apr;165(7):2167-77. doi: 10.1111/j.1476-5381.2011.01674.x. Br J Pharmacol. 2012. PMID: 21943094 Free PMC article.
-
Spider-venom peptides that target voltage-gated sodium channels: pharmacological tools and potential therapeutic leads.Toxicon. 2012 Sep 15;60(4):478-91. doi: 10.1016/j.toxicon.2012.04.337. Epub 2012 Apr 20. Toxicon. 2012. PMID: 22543187 Review.
-
Insect-selective spider toxins targeting voltage-gated sodium channels.Toxicon. 2007 Mar 15;49(4):490-512. doi: 10.1016/j.toxicon.2006.11.027. Epub 2006 Dec 5. Toxicon. 2007. PMID: 17223149 Review.
Cited by
-
Dual regulation of Kv7.2/7.3 channels by long-chain n-alcohols.J Gen Physiol. 2023 Feb 6;155(2):e202213191. doi: 10.1085/jgp.202213191. Epub 2022 Dec 19. J Gen Physiol. 2023. PMID: 36534082 Free PMC article.
-
Alcohol's effects on lipid bilayer properties.Biophys J. 2011 Aug 17;101(4):847-55. doi: 10.1016/j.bpj.2011.07.013. Biophys J. 2011. PMID: 21843475 Free PMC article.
-
Problematic alcohol use associates with sodium channel and clathrin linker 1 (SCLT1) in trauma-exposed populations.Addict Biol. 2018 Sep;23(5):1145-1159. doi: 10.1111/adb.12569. Epub 2017 Oct 30. Addict Biol. 2018. PMID: 29082582 Free PMC article.
-
Comparative effects of halogenated inhaled anesthetics on voltage-gated Na+ channel function.Anesthesiology. 2009 Mar;110(3):582-90. doi: 10.1097/ALN.0b013e318197941e. Anesthesiology. 2009. PMID: 19225394 Free PMC article.
-
Mechanisms of inhibition of CaV3.1 T-type calcium current by aliphatic alcohols.Neuropharmacology. 2010 Jul-Aug;59(1-2):58-69. doi: 10.1016/j.neuropharm.2010.03.016. Epub 2010 Apr 2. Neuropharmacology. 2010. PMID: 20363234 Free PMC article.
References
-
- Catterall WA. From ionic currents to molecular mechanisms: The structure and function of voltage-gated sodium channels. Neuron. 2000;26:13–25. - PubMed
-
- Catterall WA, Goldin AL, Waxman SG. International Union of Pharmacology. XLVII. Nomenclature and structure-function relationships of voltage-gated sodium channels. Pharmacol Rev. 2005;57:397–409. - PubMed
-
- Cordeiro JM, Barajas-Martinez H, Hong K, Burashnikov E, Pfeiffer R, Orsino AM, Wu YS, Hu D, Brugada J, Brugada P, Antzelevitch C, Dumaine R, Brugada R. Compound heterozygous mutations P336L and I1660V in the human cardiac sodium channel associated with the Brugada syndrome. Circulation. 2006;114:2026–2033. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

