Global regulation of the interphase microtubule system by abundantly expressed Op18/stathmin
- PMID: 18434595
- PMCID: PMC2441667
- DOI: 10.1091/mbc.e08-01-0058
Global regulation of the interphase microtubule system by abundantly expressed Op18/stathmin
Abstract
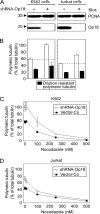
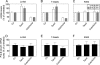
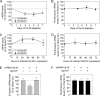
Op18/stathmin (Op18), a conserved microtubule-depolymerizing and tubulin heterodimer-binding protein, is a major interphase regulator of tubulin monomer-polymer partitioning in diverse cell types in which Op18 is abundant. Here, we addressed the question of whether the microtubule regulatory function of Op18 includes regulation of tubulin heterodimer synthesis. We used two human cell model systems, K562 and Jurkat, combined with strategies for regulatable overexpression or depletion of Op18. Although Op18 depletion caused extensive overpolymerization and increased microtubule content in both cell types, we did not detect any alteration in polymer stability. Interestingly, however, we found that Op18 mediates positive regulation of tubulin heterodimer content in Jurkat cells, which was not observed in K562 cells. By analysis of cells treated with microtubule-poisoning drugs, we found that Jurkat cells regulate tubulin mRNA levels by a posttranscriptional mechanism similarly to normal primary cells, whereas this mechanism is nonfunctional in K562 cells. We present evidence that Op18 mediates posttranscriptional regulation of tubulin mRNA in Jurkat cells through the same basic autoregulatory mechanism as microtubule-poisoning drugs. This, combined with potent regulation of tubulin monomer-polymer partitioning, enables Op18 to exert global regulation of the microtubule system.
Figures





Similar articles
-
Deciphering the cellular functions of the Op18/Stathmin family of microtubule-regulators by plasma membrane-targeted localization.Mol Biol Cell. 2003 Sep;14(9):3716-29. doi: 10.1091/mbc.e03-03-0126. Epub 2003 May 29. Mol Biol Cell. 2003. PMID: 12972559 Free PMC article.
-
Interphase-specific phosphorylation-mediated regulation of tubulin dimer partitioning in human cells.Mol Biol Cell. 2007 May;18(5):1909-17. doi: 10.1091/mbc.e07-01-0019. Epub 2007 Mar 7. Mol Biol Cell. 2007. PMID: 17344472 Free PMC article.
-
Op18/Stathmin counteracts the activity of overexpressed tubulin-disrupting proteins in a human leukemia cell line.Exp Cell Res. 2008 Apr 1;314(6):1367-77. doi: 10.1016/j.yexcr.2007.12.018. Epub 2008 Jan 5. Exp Cell Res. 2008. PMID: 18262179
-
Predominant regulators of tubulin monomer-polymer partitioning and their implication for cell polarization.Cell Mol Life Sci. 2009 Oct;66(20):3263-76. doi: 10.1007/s00018-009-0084-5. Epub 2009 Jul 8. Cell Mol Life Sci. 2009. PMID: 19585080 Free PMC article. Review.
-
The oncoprotein 18/stathmin family of microtubule destabilizers.Curr Opin Cell Biol. 2002 Feb;14(1):18-24. doi: 10.1016/s0955-0674(01)00289-7. Curr Opin Cell Biol. 2002. PMID: 11792540 Review.
Cited by
-
The microtubule cytoskeleton is required for a G2 cell cycle delay in cancer cells lacking stathmin and p53.Cytoskeleton (Hoboken). 2012 May;69(5):278-89. doi: 10.1002/cm.21024. Epub 2012 Mar 29. Cytoskeleton (Hoboken). 2012. PMID: 22407961 Free PMC article.
-
The Microtubule Regulatory Protein Stathmin Is Required to Maintain the Integrity of Axonal Microtubules in Drosophila.PLoS One. 2013 Jun 26;8(6):e68324. doi: 10.1371/journal.pone.0068324. Print 2013. PLoS One. 2013. PMID: 23840848 Free PMC article.
-
Deciphering the rules governing assembly order of mammalian septin complexes.Mol Biol Cell. 2011 Sep;22(17):3152-64. doi: 10.1091/mbc.E11-03-0253. Epub 2011 Jul 7. Mol Biol Cell. 2011. PMID: 21737677 Free PMC article.
-
STAT3-stathmin interactions control microtubule dynamics in migrating T-cells.J Biol Chem. 2009 May 1;284(18):12349-62. doi: 10.1074/jbc.M807761200. Epub 2009 Feb 26. J Biol Chem. 2009. PMID: 19251695 Free PMC article.
-
c-Jun N-terminal kinase phosphorylation of stathmin confers protection against cellular stress.J Biol Chem. 2010 Sep 10;285(37):29001-13. doi: 10.1074/jbc.M110.128454. Epub 2010 Jul 14. J Biol Chem. 2010. PMID: 20630875 Free PMC article.
References
-
- Barlow S. B., Gonzalez-Garay M. L., Cabral F. Paclitaxel-dependent mutants have severely reduced microtubule assembly and reduced tubulin synthesis. J. Cell Sci. 2002;115:3469–3478. - PubMed
-
- Belmont L. D., Mitchison T. J. Identification of a protein that interacts with tubulin dimers and increases the catastrophe rate of microtubules. Cell. 1996;84:623–631. - PubMed
-
- Brattsand G., Roos G., Marklund U., Ueda H., Landberg G., Nanberg E., Sideras P., Gullberg M. Quantitative analysis of the expression and regulation of an activation-regulated phosphoprotein (oncoprotein 18) in normal and neoplastic cells. Leukemia. 1993;7:569–579. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

