Functional interaction of regulatory factors with the Pgc-1alpha promoter in response to exercise by in vivo imaging
- PMID: 18434626
- PMCID: PMC2493547
- DOI: 10.1152/ajpcell.00104.2008
Functional interaction of regulatory factors with the Pgc-1alpha promoter in response to exercise by in vivo imaging
Abstract
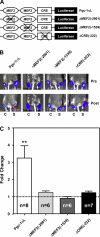
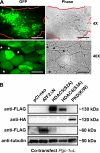
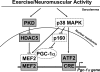
Real-time optical bioluminescence imaging is a powerful tool for studies of gene regulation in living animals. To elucidate exercise-induced signaling/transcriptional control of the peroxisome proliferator-activated receptor-gamma coactivator-1alpha (Pgc-1alpha) gene in skeletal muscle, we combined this technology with electric pulse-mediated gene transfer to cotransfect the Pgc-1alpha reporter gene with plasmid DNA encoding mutant/deletion forms of putative regulatory factors and, thereby, assess the responsiveness of the promoter to skeletal muscle contraction. We show that each of the myocyte enhancer factor 2 sites on the Pgc-1alpha promoter is required for contractile activity-induced Pgc-1alpha transcription. The responsiveness of the Pgc-1alpha promoter to contractile activity could be completely blocked by overexpression of the dominant-negative form of activating transcription factor 2 (ATF2), the signaling-resistant form of histone deacetylase (HDAC) 5 (HDAC5), or protein kinase D (PKD), but not by HDAC4. These findings provide in vivo evidence for functional interactions between PKD/HDAC5 and ATF2 regulatory factors and the Pgc-1alpha gene in adult skeletal muscle.
Figures

References
-
- Akimoto T, Pohnert SC, Li P, Zhang M, Gumbs C, Rosenberg PB, Williams RS, Yan Z. Exercise stimulates Pgc-1α transcription in skeletal muscle through activation of the p38 MAPK pathway. J Biol Chem 280: 19587–19593, 2005. - PubMed
-
- Akimoto T, Sorg BS, Yan Z. Real-time imaging of peroxisome proliferator-activated receptor-γ coactivator-1α promoter activity in skeletal muscles of living mice. Am J Physiol Cell Physiol 287: C790–C796, 2004. - PubMed
-
- Baar K, Wende AR, Jones TE, Marison M, Nolte LA, Chen M, Kelly DP, Holloszy JO. Adaptations of skeletal muscle to exercise: rapid increase in the transcriptional coactivator PGC-1. FASEB J 16: 1879–1886, 2002. - PubMed
-
- Boppart MD, Hirshman MF, Sakamoto K, Fielding RA, Goodyear LJ. Static stretch increases c-Jun NH2-terminal kinase activity and p38 phosphorylation in rat skeletal muscle. Am J Physiol Cell Physiol 280: C352–C358, 2001. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

