Contaminated heparin associated with adverse clinical events and activation of the contact system
- PMID: 18434646
- PMCID: PMC3778681
- DOI: 10.1056/NEJMoa0803200
Contaminated heparin associated with adverse clinical events and activation of the contact system
Erratum in
- N Engl J Med. 2010 Mar 18;362(11):1056
Abstract
Background: There is an urgent need to determine whether oversulfated chondroitin sulfate (OSCS), a compound contaminating heparin supplies worldwide, is the cause of the severe anaphylactoid reactions that have occurred after intravenous heparin administration in the United States and Germany.
Methods: Heparin procured from the Food and Drug Administration, consisting of suspect lots of heparin associated with the clinical events as well as control lots of heparin, were screened in a blinded fashion both for the presence of OSCS and for any biologic activity that could potentially link the contaminant to the observed clinical adverse events. In vitro assays for the activation of the contact system and the complement cascade were performed. In addition, the ability of OSCS to recapitulate key clinical manifestations in vivo was tested in swine.
Results: The OSCS found in contaminated lots of unfractionated heparin, as well as a synthetically generated OSCS reference standard, directly activated the kinin-kallikrein pathway in human plasma, which can lead to the generation of bradykinin, a potent vasoactive mediator. In addition, OSCS induced generation of C3a and C5a, potent anaphylatoxins derived from complement proteins. Activation of these two pathways was unexpectedly linked and dependent on fluid-phase activation of factor XII. Screening of plasma samples from various species indicated that swine and humans are sensitive to the effects of OSCS in a similar manner. OSCS-containing heparin and synthetically derived OSCS induced hypotension associated with kallikrein activation when administered by intravenous infusion in swine.
Conclusions: Our results provide a scientific rationale for a potential biologic link between the presence of OSCS in suspect lots of heparin and the observed clinical adverse events. An assay to assess the amidolytic activity of kallikrein can supplement analytic tests to protect the heparin supply chain by screening for OSCS and other highly sulfated polysaccharide contaminants of heparin that can activate the contact system.
Copyright 2008 Massachusetts Medical Society.
Conflict of interest statement
No other potential conflict of interest relevant to this article was reported.
Figures

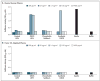
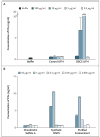

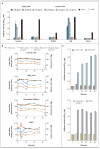
Comment in
-
Coagulation and adulteration--building on science and policy lessons from 1905.N Engl J Med. 2008 Jun 5;358(23):2429-31. doi: 10.1056/NEJMp0803685. N Engl J Med. 2008. PMID: 18525040 No abstract available.
-
Heparin comes clean.N Engl J Med. 2008 Jun 5;358(23):2505-9. doi: 10.1056/NEJMe0803599. N Engl J Med. 2008. PMID: 18525048 No abstract available.
-
Contaminated heparin.N Engl J Med. 2008 Sep 18;359(12):1291-2; author reply 1293. doi: 10.1056/NEJMc081387. N Engl J Med. 2008. PMID: 18799565 No abstract available.
-
Contaminated heparin.N Engl J Med. 2008 Sep 18;359(12):1292-3; author reply 1293. N Engl J Med. 2008. PMID: 18810795 No abstract available.
-
Contaminated heparin preparations, severe adverse events and the contact system.Clin Appl Thromb Hemost. 2008 Oct;14(4):489-91. doi: 10.1177/1076029608324291. Clin Appl Thromb Hemost. 2008. PMID: 18815138 No abstract available.
References
-
- Acute allergic-type reactions among patients undergoing hemodialysis — multiple states, 2007–2008. MMWR Morb Mortal Wkly Rep. 2008;57:124–5. - PubMed
-
- Information on adverse event reports and heparin. Rockville, MD: Food and Drug Administration; 2008. [Accessed May 12, 2008]. at http://www.fda.gov/cder/drug/infopage/heparin/adverse_events.htm.
-
- Information on heparin sodium injection. Rockville, MD: Food and Drug Administration; 2008. [Accessed May 12,2008]. at http://www.fda.gov/cder/drug/infopage/heparin/default.htm#screening.
-
- Hojima Y, Cochrane CG, Wiggins RC, Austen KF, Stevens RL. In vitro activation of the contact (Hageman factor) system of plasma by heparin and chondroitin sulfate E. Blood. 1984;63:1453–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
