Identification of a new class of nonpeptidic inhibitors of cruzain
- PMID: 18435536
- PMCID: PMC2765048
- DOI: 10.1021/ja710254m
Identification of a new class of nonpeptidic inhibitors of cruzain
Abstract
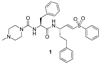
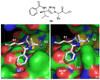
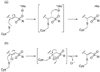
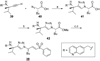
Cruzain is the major cysteine protease of Trypanosoma cruzi, which is the causative agent of Chagas disease and is a promising target for the development of new chemotherapy. With the goal of developing potent nonpeptidic inhibitors of cruzain, the substrate activity screening (SAS) method was used to screen a library of protease substrates initially designed to target the homologous human protease cathepsin S. Structure-based design was next used to further improve substrate cleavage efficiency by introducing additional binding interactions in the S3 pocket of cruzain. The optimized substrates were then converted to inhibitors by the introduction of cysteine protease mechanism-based pharmacophores. Inhibitor 38 was determined to be reversible even though it incorporated the vinyl sulfone pharmacophore that is well documented to give irreversible cruzain inhibition for peptidic inhibitors. The previously unexplored beta-chloro vinyl sulfone pharmacophore provided mechanistic insight that led to the development of potent irreversible acyl- and aryl-oxymethyl ketone cruzain inhibitors. For these inhibitors, potency did not solely depend on leaving group p K a, with 2,3,5,6-tetrafluorophenoxymethyl ketone 54 identified as one of the most potent inhibitors with a second-order inactivation constant of 147,000 s (-1) M (-1). This inhibitor completely eradicated the T. cruzi parasite from mammalian cell cultures and consequently has the potential to lead to new chemotherapeutics for Chagas disease.
Figures








Similar articles
-
Crystal structures of reversible ketone-Based inhibitors of the cysteine protease cruzain.Bioorg Med Chem. 2003 Jan 2;11(1):21-9. doi: 10.1016/s0968-0896(02)00427-3. Bioorg Med Chem. 2003. PMID: 12467703
-
Nonpeptidic tetrafluorophenoxymethyl ketone cruzain inhibitors as promising new leads for Chagas disease chemotherapy.J Med Chem. 2010 Feb 25;53(4):1763-73. doi: 10.1021/jm901633v. J Med Chem. 2010. PMID: 20088534 Free PMC article.
-
In vitro and in vivo studies of the trypanocidal properties of WRR-483 against Trypanosoma cruzi.PLoS Negl Trop Dis. 2010 Sep 14;4(9):e825. doi: 10.1371/journal.pntd.0000825. PLoS Negl Trop Dis. 2010. PMID: 20856868 Free PMC article.
-
Synthesis and structure-activity relationship studies of cruzain and rhodesain inhibitors.Eur J Med Chem. 2018 Sep 5;157:1426-1459. doi: 10.1016/j.ejmech.2018.08.079. Epub 2018 Aug 31. Eur J Med Chem. 2018. PMID: 30282318 Review.
-
Cruzain : the path from target validation to the clinic.Adv Exp Med Biol. 2011;712:100-15. doi: 10.1007/978-1-4419-8414-2_7. Adv Exp Med Biol. 2011. PMID: 21660661 Review.
Cited by
-
Emerging principles in protease-based drug discovery.Nat Rev Drug Discov. 2010 Sep;9(9):690-701. doi: 10.1038/nrd3053. Nat Rev Drug Discov. 2010. PMID: 20811381 Free PMC article. Review.
-
Synthesis of a sugar-based thiosemicarbazone series and structure-activity relationship versus the parasite cysteine proteases rhodesain, cruzain, and Schistosoma mansoni cathepsin B1.Antimicrob Agents Chemother. 2015 May;59(5):2666-77. doi: 10.1128/AAC.04601-14. Epub 2015 Feb 23. Antimicrob Agents Chemother. 2015. PMID: 25712353 Free PMC article.
-
Two approaches to discovering and developing new drugs for Chagas disease.Mem Inst Oswaldo Cruz. 2009 Jul;104 Suppl 1(0 1):263-9. doi: 10.1590/s0074-02762009000900034. Mem Inst Oswaldo Cruz. 2009. PMID: 19753483 Free PMC article.
-
Novel coumarins active against Trypanosoma cruzi and toxicity assessment using the animal model Caenorhabditis elegans.BMC Pharmacol Toxicol. 2019 Dec 19;20(Suppl 1):76. doi: 10.1186/s40360-019-0357-z. BMC Pharmacol Toxicol. 2019. PMID: 31852548 Free PMC article.
-
Coadministration of cruzipain and GM-CSF DNAs, a new immunotherapeutic vaccine against Trypanosoma cruzi infection.Hum Vaccin Immunother. 2016;12(2):438-50. doi: 10.1080/21645515.2015.1078044. Hum Vaccin Immunother. 2016. PMID: 26312947 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

