Ethanol sensitivity and tolerance in long-term memory mutants of Drosophila melanogaster
- PMID: 18435628
- PMCID: PMC3044939
- DOI: 10.1111/j.1530-0277.2008.00659.x
Ethanol sensitivity and tolerance in long-term memory mutants of Drosophila melanogaster
Abstract
Background: It has become increasingly clear that molecular and neural mechanisms underlying learning and memory and drug addiction are largely shared. To confirm and extend these findings, we analyzed ethanol-responsive behaviors of a collection of Drosophila long-term memory mutants.
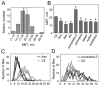
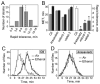
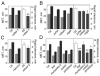
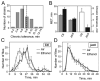
Methods: For each mutant, sensitivity to the acute uncoordinating effects of ethanol was quantified using the inebriometer. Additionally, 2 distinct forms of ethanol tolerance were measured: rapid tolerance, which develops in response to a single brief exposure to a high concentration of ethanol vapor; and chronic tolerance, which develops following a sustained low-level exposure.
Results: Several mutants were identified with altered sensitivity, rapid or chronic tolerance, while a number of mutants exhibited multiple defects.
Conclusions: The corresponding genes in these mutants represent areas of potential overlap between learning and memory and behavioral responses to alcohol. These genes also define components shared between different ethanol behavioral responses.
Figures


References
-
- Arquier N, Bourouis M, Colombani J, Leopold P. Drosophila Lk6 kinase controls phosphorylation of eukaryotic translation initiation factor 4E and promotes normal growth and development. Curr Biol. 2005;15(1):19–23. - PubMed
-
- Bennett B, Downing C, Carosone-Link P, Ponicsan H, Ruf C, Johnson TE. Quantitative trait locus mapping for acute functional tolerance to ethanol in the L × S recombinant inbred panel. Alcohol Clin Exp Res. 2007;31(2):200–208. - PubMed
-
- Berger KH, Heberlein U, Moore MS. Rapid and chronic: two distinct forms of ethanol tolerance in Drosophila. Alcohol Clin Exp Res. 2004;28(10):1469–1480. - PubMed
-
- Bitran M, Kalant H. Effect of anisomycin on the development of rapid tolerance to ethanol-induced motor impairment. Pharmacol Biochem Behav. 1993;45(1):225–228. - PubMed
-
- Campbell JL, Nash HA. Volatile general anesthetics reveal a neurobiological role for the white and brown genes of Drosophila melanogaster. J Neurobiol. 2001;49(4):339–349. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

