Hydrogen peroxide promotes Abeta production through JNK-dependent activation of gamma-secretase
- PMID: 18436531
- PMCID: PMC2427353
- DOI: 10.1074/jbc.M800013200
Hydrogen peroxide promotes Abeta production through JNK-dependent activation of gamma-secretase
Abstract
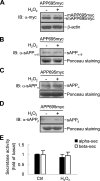
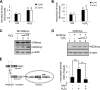
Accumulation of senile plaques composed of amyloid beta-peptide (Abeta) is a pathological hallmark of Alzheimer disease (AD), and Abeta is generated through the sequential cleavage of amyloid precursor protein (APP) by beta- and gamma-secretase. Although oxidative stress has been implicated in the AD pathogenesis by inducing Abeta production, the underlying mechanism remains elusive. Here we show that the pro-oxidant H(2)O(2) promotes Abeta production through c-Jun N-terminal kinase (JNK)-dependent activation of gamma-secretase. Treatment with H(2)O(2) induced significant increase in the levels of intracellular and secreted Abeta in human neuroblastoma SH-SY5Y cells. Although gamma-secretase-mediated cleavage of APP or C99 was enhanced upon H(2)O(2) treatment, expression of APP or its alpha/beta-secretase-mediated cleavage was not affected. Silencing of the stress-activated JNK by small interfering RNA or the specific JNK inhibitor SP600125 reduced H(2)O(2)-induced gamma-secretase-mediated cleavage of APP. JNK activity was augmented in human brain tissues from AD patients and active JNK located surrounding the senile plaques in the brain of AD model mouse. Our data suggest that oxidative stress-activated JNK may contribute to senile plaque expansion through the promotion of gamma-secretase-mediated APP cleavage and Abeta production.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

