Colony-stimulating factor-1 transfection of myoblasts improves the repair of failing myocardium following autologous myoblast transplantation
- PMID: 18436538
- PMCID: PMC2732060
- DOI: 10.1093/cvr/cvn097
Colony-stimulating factor-1 transfection of myoblasts improves the repair of failing myocardium following autologous myoblast transplantation
Abstract
Aims: Skeletal myoblasts are used in repair of ischaemic myocardium. However, a large fraction of grafted myoblasts degenerate upon engraftment. Colony-stimulating factor-1 (CSF-1) accelerates myoblast proliferation and angiogenesis. We hypothesized that CSF-1 overexpression improves myoblast survival and cardiac function in ischaemia-induced heart failure.
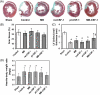
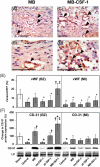
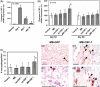
Methods and results: Three weeks following myocardial infarction, rats developed heart failure and received intramyocardial injections of mouse CSF-1-transfected or untransfected primary autologous rat myoblasts, recombinant human CSF-1, mouse CSF-1 expressing plasmids, or culture medium. Tissue gene and protein expression was measured by quantitative RT-PCR (reverse transcription-polymerase chain reaction) and western blotting. Fluorescence imaging and immunocytochemistry were used to analyse myoblasts, endothelial cells, macrophages, and infarct wall thickening. Electrocardiograms were recorded online using a telemetry system. Left ventricular function was assessed by echocardiography over time, and improved significantly only in the CSF-1-overexpressing myoblast group. CSF-1-overexpression enhanced myoblast numbers and was associated with an increased infarct wall thickness, enhanced angiogenesis, increased macrophage recruitment and upregulated matrix metalloproteases (MMP)-2 and -12 in the zone bordering the infarction. Transplantation of CSF-1-overexpressing myoblasts did not result in major arrhythmias.
Conclusion: Autologous intramyocardial transplantation of CSF-1 overexpressing myoblasts might be a novel strategy in the treatment of ischaemia-induced heart failure.
Figures



Comment in
-
Towards the second generation of skeletal myoblasts?Cardiovasc Res. 2008 Aug 1;79(3):355-6. doi: 10.1093/cvr/cvn132. Epub 2008 May 28. Cardiovasc Res. 2008. PMID: 18508855 No abstract available.
References
-
- Menasche P, Hagege AA, Vilquin JT, Desnos M, Abergel E, Pouzet B, et al. Autologous skeletal myoblast transplantation for severe postinfarction left ventricular dysfunction. J Am Coll Cardiol. 2003;41:1078–1083. - PubMed
-
- Taylor DA, Atkins BZ, Hungspreugs P, Jones TR, Reedy MC, Hutcheson KA, et al. Regenerating functional myocardium: improved performance after skeletal myoblast transplantation. Nat Med. 1998;4:929–933. - PubMed
-
- Jain M, DerSimonian H, Brenner DA, Ngoy S, Teller P, Edge AS, et al. Cell therapy attenuates deleterious ventricular remodeling and improves cardiac performance after myocardial infarction. Circulation. 2001;103:1920–1927. - PubMed
-
- Menasche P, Hagege AA, Scorsin M, Pouzet B, Desnos M, Duboc D, et al. Myoblast transplantation for heart failure. Lancet. 2001;357:279–280. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

