Genetic approaches identify adult pituitary stem cells
- PMID: 18436641
- PMCID: PMC2359820
- DOI: 10.1073/pnas.0801644105
Genetic approaches identify adult pituitary stem cells
Erratum in
- Proc Natl Acad Sci U S A. 2008 Aug 5;105(31):11032
Abstract
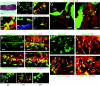
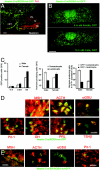
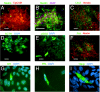
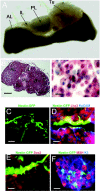
Adult tissues undergo continuous cell turnover in response to stress, damage, or physiological demand. New differentiated cells are generated from dedicated or facultative stem cells or from self-renewing differentiated cells. Here we describe a different stem cell strategy for tissue maintenance, distinct from that observed for dedicated or facultative stem cells. We report the presence of nestin-expressing adult stem cells in the perilumenal region of the mature anterior pituitary and, using genetic inducible fate mapping, demonstrate that they serve to generate subsets of all six terminally differentiated endocrine cell types of the pituitary gland. These stem cells, while not playing a significant role in organogenesis, undergo postnatal expansion and start producing differentiated progeny, which colonize the organ that initially entirely consisted of differentiated cells derived from embryonic precursors. This generates a mosaic organ with two phenotypically similar subsets of endocrine cells that have different origins and different life histories. These parallel but distinct lineages of differentiated cells in the gland may help the maturing organism adapt to changes in the metabolic regulatory landscape.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Morrison SJ, Kimble J. Asymmetric and symmetric stem-cell divisions in development and cancer. Nature. 2006;441:1068–1074. - PubMed
-
- Rawlins EL, Hogan BL. Epithelial stem cells of the lung: Privileged few or opportunities for many? Development. 2006;133:2455–2465. - PubMed
-
- Rosenfeld MG, et al. Multistep signaling and transcriptional requirements for pituitary organogenesis in vivo. Recent Prog Horm Res. 2000;55:1–13. discussion 13–14. - PubMed
-
- Scully KM, Rosenfeld MG. Pituitary development: Regulatory codes in mammalian organogenesis. Science. 2002;295:2231–2235. - PubMed
-
- Carbajo-Perez E, Watanabe YG. Cellular proliferation in the anterior pituitary of the rat during the postnatal period. Cell Tissue Res. 1990;261:333–338. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

