Thrombocytopenia and platelet abnormalities in high-density lipoprotein receptor-deficient mice
- PMID: 18436807
- PMCID: PMC2683374
- DOI: 10.1161/ATVBAHA.108.162347
Thrombocytopenia and platelet abnormalities in high-density lipoprotein receptor-deficient mice
Abstract
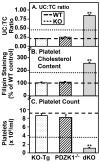
Objective: High-density lipoprotein (HDL) receptor, scavenger receptor class B, type I (SR-BI), mediated cellular uptake of lipoprotein cholesterol controls HDL structure and plasma HDL and biliary cholesterol levels. In SR-BI knockout (KO) mice, an unusually high plasma unesterified-to-total cholesterol ratio (UC:TC) and abnormally large HDL particles apparently contribute to pathology, including female infertility, susceptibility to atherosclerosis and coronary heart disease, and anemia. Here we examined the influence of SR-BI deficiency on platelets.
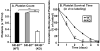
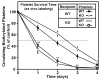
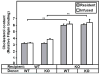
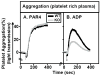
Methods and results: The high plasma UC:TC ratio in SR-BI KO mice was correlated with platelet abnormalities, including high cholesterol content, abnormal morphologies, high clearance rates, and thrombocytopenia. One day after platelets from wild-type mice were infused into SR-BI KO mice, they exhibited abnormally high cholesterol content and clearance rates similar to those of endogenous platelets. Platelets from SR-BI KO mice exhibited in vitro a blunted aggregation response to the agonist ADP but a normal response to PAR4.
Conclusions: In SR-BI KO mice abnormal circulating lipoproteins, particularly their high UC:TC ratio-rather than the absence of SR-BI in platelets themselves-induce defects in platelet structure and clearance, together with a mild defect in function.
Conflict of interest statement
Figures

References
-
- Acton S, Rigotti A, Landschulz KT, Xu S, Hobbs HH, Krieger M. Identification of scavenger receptor SR-BI as a high density lipoprotein receptor. Science. 1996;271:518–520. - PubMed
-
- Rigotti A, Miettinen HE, Krieger M. The role of the high-density lipoprotein receptor SR-BI in the lipid metabolism of endocrine and other tissues. Endocr Rev. 2003;24:357–387. - PubMed
-
- Zannis VI, Chroni A, Krieger M. Role of apoA-I, ABCA1, LCAT, and SR-BI in the biogenesis of HDL. J Mol Med. 2006;84:276–294. - PubMed
-
- Kocher O, Yesilaltay A, Cirovic C, Pal R, Rigotti A, Krieger M. Targeted disruption of the PDZK1 gene in mice causes tissue-specific depletion of the high density lipoprotein receptor scavenger receptor class B type I and altered lipoprotein metabolism. J Biol Chem. 2003;278:52820–52825. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

