IFATS collection: The role of human adipose-derived stromal cells in inflammatory microvascular remodeling and evidence of a perivascular phenotype
- PMID: 18436860
- PMCID: PMC2672107
- DOI: 10.1634/stemcells.2008-0030
IFATS collection: The role of human adipose-derived stromal cells in inflammatory microvascular remodeling and evidence of a perivascular phenotype
Abstract
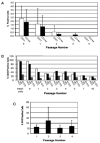
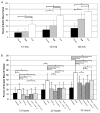
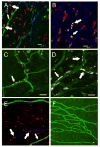
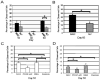
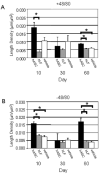
A growing body of literature suggests that human adipose-derived stromal cells (hASCs) possess developmental plasticity both in vitro and in vivo, and might represent a viable cell source for therapeutic angiogenesis and tissue engineering. We investigate their phenotypic similarity to perivascular cell types, ability to contribute to in vivo microvascular remodeling, and ability to modulate vascular stability. We evaluated hASC surface expression of vascular and stem/progenitor cell markers in vitro, as well as any effects of platelet-derived growth factor B chain (PDGF-BB) and vascular endothelial growth factor 165 on in vitro hASC migration. To ascertain in vivo behavior of hASCs in an angiogenic environment, hASCs were isolated, expanded in culture, labeled with a fluorescent marker, and injected into adult nude rat mesenteries that were stimulated to undergo microvascular remodeling. Ten, 30, and 60 days after injection, tissues from anesthetized animals were harvested and processed with immunohistochemical techniques to determine hASC quantity, positional fate in relation to microvessels, and expression of endothelial and perivascular cell markers. After 60 days, 29% of hASCs exhibited perivascular morphologies compared with 11% of injected human lung fibroblasts. hASCs exhibiting perivascular morphologies also expressed markers characteristic of vascular pericytes: smooth muscle alpha-actin (10%) and neuron-glia antigen 2 (8%). In tissues treated with hASCs, vascular density was significantly increased over age-matched controls lacking hASCs. This study demonstrates that hASCs express pericyte lineage markers in vivo and in vitro, exhibit increased migration in response to PDGF-BB in vitro, exhibit perivascular morphology when injected in vivo, and contribute to increases in microvascular density during angiogenesis by migrating toward vessels. Disclosure of potential conflicts of interest is found at the end of this article.
Figures

References
-
- Iba O, Matsubara H, Nozawa Y, et al. Angiogenesis by implantation of peripheral blood mononuclear cells and platelets into ischemic limbs. Circulation. 2002;106:2019–2025. - PubMed
-
- Peichev M, Naiyer AJ, Zhu Z, et al. Expression of VEGFR-2 and AC133 by circulating human CD34+ cells identifies a population of functional endothelial precursors. Hemostas, Thombosis, and Vasc Biol. 2000;95:952–958. - PubMed
-
- Asahara T, Haruchika M, Tomono T, et al. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res. 1999;85:221–228. - PubMed
-
- Carmeliet P, Luttun A. The role of the bone marrow-derived stem cells in (therapeutic) angiogenesis. Thrombos & Haemostas. 2001;86:289–297. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

