Validity, reliability, and feasibility of durometer measurements of scleroderma skin disease in a multicenter treatment trial
- PMID: 18438905
- PMCID: PMC3887555
- DOI: 10.1002/art.23564
Validity, reliability, and feasibility of durometer measurements of scleroderma skin disease in a multicenter treatment trial
Abstract
Objective: To determine the validity, reliability, and feasibility of durometer measurements of skin hardness as an outcome measure in clinical trials of scleroderma.
Methods: Skin hardness was measured during a multicenter treatment trial for scleroderma using handheld digital durometers with a continuous scale. Skin thickness was measured by modified Rodnan skin score (MRSS). Other outcome data collected included the Scleroderma Health Assessment Questionnaire. In a reliability exercise in advance of the trial, 9 investigators examined the same 5 scleroderma patients by MRSS and durometry.
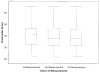
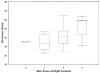
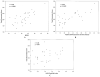
Results: Forty-three patients with early diffuse cutaneous systemic sclerosis were studied at 11 international centers (mean age 49 years [range 24-76], median disease duration 6.4 months [range 0.3-23], and median baseline MRSS 22 [range 11-38]). The reliability of durometer measurements was excellent, with high interobserver intraclass correlation coefficients (ICCs) (0.82-0.92), and each result was greater than the corresponding skin site ICCs for MRSS (0.54-0.85). Baseline durometer scores correlated well with MRSS (r = 0.69, P < 0.0001), patient self-assessments of skin disease (r = 0.69, P < 0.0001), and Health Assessment Questionnaire (HAQ) disability scores (r = 0.34, P = 0.03). Change in durometer scores correlated with change in MRSS (r = 0.70, P < 0.0001), change in patient self-assessments of skin disease (r = 0.52, P = 0.003), and change in HAQ disability scores (r = 0.42, P = 0.017). The effect size was greater for durometry than for MRSS or patient self-assessment.
Conclusion: Durometer measurements of skin hardness in patients with scleroderma are reliable, simple, accurate, demonstrate good sensitivity to change compared with traditional skin scoring, and reflect patients' self-assessments of their disease. Durometer measurements are valid, objective, and scalable, and should be considered for use as a complementary outcome measure to skin scoring in clinical trials of scleroderma.
Figures

References
-
- Clements PJ, Lachenbruch PA, Ng SC, Simmons M, Sterz M, Furst DE. Skin score: a semiquantitative measure of cutaneous involvement that improves prediction of prognosis in systemic sclerosis. Arthritis Rheum. 1990;33:1256–63. - PubMed
-
- Clements P, Lachenbruch P, Seibold J, White B, Weiner S, Martin R, et al. Inter and intraobserver variability of total skin thickness score (modified Rodnan TSS) in systemic sclerosis. J Rheumatol. 1995;22:1281–5. - PubMed
-
- Merkel PA, Clements PJ, Reveille JD, Suarez-Almazor ME, Valentini G, Furst DE. Current status of outcome measure development for clinical trials in systemic sclerosis: report from OMERACT 6. J Rheumatol. 2003;30:1630–47. - PubMed
-
- Kissin EY, Schiller AM, Gelbard RB, Anderson JJ, Falanga V, Simms RW, et al. Durometry for the assessment of skin disease in systemic sclerosis. Arthritis Rheum. 2006;55:603–9. - PubMed
-
- Kissin EY, Merkel PA, Lafyatis R. Myofibroblasts and hyalinized collagen as markers of skin disease in systemic sclerosis. Arthritis Rheum. 2006;54:3655–60. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

