Effect of HIV-1-related protein expression on cardiac and skeletal muscles from transgenic rats
- PMID: 18439274
- PMCID: PMC2365956
- DOI: 10.1186/1742-6405-5-8
Effect of HIV-1-related protein expression on cardiac and skeletal muscles from transgenic rats
Abstract
Background: Human immunodeficiency virus type 1 (HIV-1) infection and the consequent acquired immunodeficiency syndrome (AIDS) has protean manifestations, including muscle wasting and cardiomyopathy, which contribute to its high morbidity. The pathogenesis of these myopathies remains partially understood, and may include nutritional deficiencies, biochemical abnormalities, inflammation, and other mechanisms due to viral infection and replication. Growing evidence has suggested that HIV-1-related proteins expressed by the host in response to viral infection, including Tat and gp120, may also be involved in the pathophysiology of AIDS, particularly in cells or tissues that are not directly infected with HIV-1. To explore the potentially independent effects of HIV-1-related proteins on heart and skeletal muscles, we used a transgenic rat model that expresses several HIV-1-related proteins (e.g., Tat, gp120, and Nef). Outcome measures included basic heart and skeletal muscle morphology, glutathione metabolism and oxidative stress, and gene expressions of atrogin-1, muscle ring finger protein-1 (MuRF-1) and Transforming Growth Factor-beta1 (TGFbeta1), three factors associated with muscle catabolism.
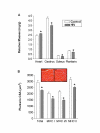
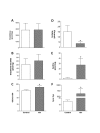
Results: Consistent with HIV-1 associated myopathies in humans, HIV-1 transgenic rats had increased relative heart masses, decreased relative masses of soleus, plantaris and gastrocnemius muscles, and decreased total and myosin heavy chain type-specific plantaris muscle fiber areas. In both tissues, the levels of cystine (Cyss), the oxidized form of the anti-oxidant cysteine (Cys), and Cyss:Cys ratios were significantly elevated, and cardiac tissue from HIV-1 transgenic rats had altered glutathione metabolism, all reflective of significant oxidative stress. In HIV-1 transgenic rat hearts, MuRF-1 gene expression was increased. Further, HIV-1-related protein expression also increased atrogin-1 (approximately 14- and approximately 3-fold) and TGFbeta1 (approximately 5-fold and approximately 3-fold) in heart and plantaris muscle tissues, respectively.
Conclusion: We provide compelling experimental evidence that HIV-1-related proteins can lead to significant cardiac and skeletal muscle complications independently of viral infection or replication. Our data support the concept that HIV-1-related proteins are not merely disease markers, but rather have significant biological activity that may lead to increased oxidative stress, the stimulation of redox-sensitive pathways, and altered muscle morphologies. If correct, this pathophysiological scheme suggests that the use of dietary thiol supplements could reduce skeletal and cardiac muscle dysfunction in HIV-1-infected individuals.
Figures


Similar articles
-
Skeletal and cardiac myopathy in HIV-1 transgenic rats.Am J Physiol Endocrinol Metab. 2008 Oct;295(4):E964-73. doi: 10.1152/ajpendo.90482.2008. Epub 2008 Aug 19. Am J Physiol Endocrinol Metab. 2008. PMID: 18713959 Free PMC article.
-
Chronic alcohol ingestion exacerbates skeletal muscle myopathy in HIV-1 transgenic rats.AIDS Res Ther. 2011 Aug 16;8:30. doi: 10.1186/1742-6405-8-30. AIDS Res Ther. 2011. PMID: 21846370 Free PMC article.
-
HIV-1 transgenic expression in mice induces selective atrophy of fast-glycolytic skeletal muscle fibers.Front Biosci. 2008 Jan 1;13:2797-805. doi: 10.2741/2886. Front Biosci. 2008. PMID: 17981754
-
The role of HIV-1 activated leukocyte adhesion mechanisms and matrix metalloproteinase secretion in AIDS pathogenesis (Review).Int J Mol Med. 1998 Feb;1(2):361-6. doi: 10.3892/ijmm.1.2.361. Int J Mol Med. 1998. PMID: 9852238 Review.
-
Role of cysteine and glutathione in HIV infection and other diseases associated with muscle wasting and immunological dysfunction.FASEB J. 1997 Nov;11(13):1077-89. doi: 10.1096/fasebj.11.13.9367343. FASEB J. 1997. PMID: 9367343 Review.
Cited by
-
Combined effects of hyperglycemic conditions and HIV-1 Nef: a potential model for induced HIV neuropathogenesis.Virol J. 2009 Oct 30;6:183. doi: 10.1186/1743-422X-6-183. Virol J. 2009. PMID: 19878567 Free PMC article.
-
Models of accelerated sarcopenia: critical pieces for solving the puzzle of age-related muscle atrophy.Ageing Res Rev. 2010 Oct;9(4):369-83. doi: 10.1016/j.arr.2010.04.004. Epub 2010 May 14. Ageing Res Rev. 2010. PMID: 20438881 Free PMC article. Review.
-
Advances in our understanding of the pathogenesis of HIV-1 associated nephropathy in children.Future Virol. 2011 Jul;6(7):883-894. doi: 10.2217/fvl.11.57. Future Virol. 2011. PMID: 22162721 Free PMC article.
-
Can the vector space model be used to identify biological entity activities?BMC Genomics. 2011 Dec 22;12 Suppl 4(Suppl 4):S1. doi: 10.1186/1471-2164-12-S4-S1. Epub 2011 Dec 22. BMC Genomics. 2011. PMID: 22369514 Free PMC article.
-
Skeletal and cardiac myopathy in HIV-1 transgenic rats.Am J Physiol Endocrinol Metab. 2008 Oct;295(4):E964-73. doi: 10.1152/ajpendo.90482.2008. Epub 2008 Aug 19. Am J Physiol Endocrinol Metab. 2008. PMID: 18713959 Free PMC article.
References
-
- Gonzalez-Cadavid NF, Taylor WE, Yarasheski K, Sinha-Hikim I, Ma K, Ezzat S, Shen R, Lalani R, Asa S, Mamita M, Nair G, Arver S, Bhasin S. Organization of the human myostatin gene and expression in healthy men and HIV-infected men with muscle wasting. Proc Natl Acad Sci USA. 1998;95:14938–14943. doi: 10.1073/pnas.95.25.14938. - DOI - PMC - PubMed
-
- Hack V, Schmid D, Breitkreutz R, Stahl-Henning C, Drings P, Kinsherf R, Taut F, Holm E, Droge W. Cystine levels, cystine flux, and protein catabolism in cancer cachexia, HIV/SIV infection, and senescence. FASEB J. 1997;11:84–92. - PubMed
-
- Patrick L. Nutrients and HIV: part three – N-acetylcysteine, alpha-lipoic acid, L-glutamine, and L-carnitine. Altern Med Rev. 2000;5:290–305. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

