HIV-1 latency in actively dividing human T cell lines
- PMID: 18439275
- PMCID: PMC2387167
- DOI: 10.1186/1742-4690-5-37
HIV-1 latency in actively dividing human T cell lines
Abstract
Background: Eradication of HIV-1 from an infected individual cannot be achieved by current drug regimens. Viral reservoirs established early during the infection remain unaffected by anti-retroviral therapy and are able to replenish systemic infection upon interruption of the treatment. Therapeutic targeting of viral latency will require a better understanding of the basic mechanisms underlying the establishment and long-term maintenance of HIV-1 in resting memory CD4 T cells, the most prominent reservoir of transcriptional silent provirus. However, the molecular mechanisms that permit long-term transcriptional control of proviral gene expression in these cells are still not well understood. Exploring the molecular details of viral latency will provide new insights for eventual future therapeutics that aim at viral eradication.
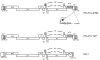
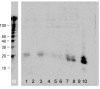
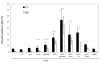
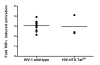
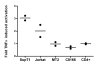
Results: We set out to develop a new in vitro HIV-1 latency model system using the doxycycline (dox)-inducible HIV-rtTA variant. Stable cell clones were generated with a silent HIV-1 provirus, which can subsequently be activated by dox-addition. Surprisingly, only a minority of the cells was able to induce viral gene expression and a spreading infection, eventhough these experiments were performed with the actively dividing SupT1 T cell line. These latent proviruses are responsive to TNFalpha treatment and alteration of the DNA methylation status with 5-Azacytidine or genistein, but not responsive to the regular T cell activators PMA and IL2. Follow-up experiments in several T cell lines and with wild-type HIV-1 support these findings.
Conclusion: We describe the development of a new in vitro model for HIV-1 latency and discuss the advantages of this system. The data suggest that HIV-1 proviral latency is not restricted to resting T cells, but rather an intrinsic property of the virus.
Figures
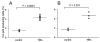
Similar articles
-
Latency: the hidden HIV-1 challenge.Retrovirology. 2006 Jan 16;3:7. doi: 10.1186/1742-4690-3-7. Retrovirology. 2006. PMID: 16412247 Free PMC article. Review.
-
A lentiviral vector that activates latent human immunodeficiency virus-1 proviruses by the overexpression of tat and that kills the infected cells.Hum Gene Ther. 2009 Nov;20(11):1259-68. doi: 10.1089/hum.2009.059. Hum Gene Ther. 2009. PMID: 19604078
-
Development of 5' LTR DNA methylation of latent HIV-1 provirus in cell line models and in long-term-infected individuals.Clin Epigenetics. 2016 Feb 19;8:19. doi: 10.1186/s13148-016-0185-6. eCollection 2016. Clin Epigenetics. 2016. PMID: 26900410 Free PMC article.
-
Posttranscriptional Regulation of HIV-1 Gene Expression during Replication and Reactivation from Latency by Nuclear Matrix Protein MATR3.mBio. 2018 Nov 13;9(6):e02158-18. doi: 10.1128/mBio.02158-18. mBio. 2018. PMID: 30425153 Free PMC article.
-
The Molecular Basis for Human Immunodeficiency Virus Latency.Annu Rev Virol. 2017 Sep 29;4(1):261-285. doi: 10.1146/annurev-virology-101416-041646. Epub 2017 Jul 17. Annu Rev Virol. 2017. PMID: 28715973 Review.
Cited by
-
Cellular and molecular mechanisms involved in the establishment of HIV-1 latency.Retrovirology. 2013 Feb 1;10:11. doi: 10.1186/1742-4690-10-11. Retrovirology. 2013. PMID: 23375003 Free PMC article. Review.
-
Progress and challenges in the use of latent HIV-1 reactivating agents.Acta Pharmacol Sin. 2015 Aug;36(8):908-16. doi: 10.1038/aps.2015.22. Epub 2015 Jun 1. Acta Pharmacol Sin. 2015. PMID: 26027656 Free PMC article. Review.
-
Intracellular transactivation of HIV can account for the decelerating decay of virus load during drug therapy.Mol Syst Biol. 2010;6:348. doi: 10.1038/msb.2010.4. Epub 2010 Feb 16. Mol Syst Biol. 2010. PMID: 20160709 Free PMC article.
-
Cell line-dependent variability in HIV activation employing DNMT inhibitors.Virol J. 2010 Oct 13;7:266. doi: 10.1186/1743-422X-7-266. Virol J. 2010. PMID: 20942961 Free PMC article.
-
Oral complications of HIV disease.Clinics (Sao Paulo). 2009 May;64(5):459-70. doi: 10.1590/s1807-59322009000500014. Clinics (Sao Paulo). 2009. PMID: 19488613 Free PMC article. Review.
References
-
- Frenkel LM, Wang Y, Learn GH, McKernan JL, Ellis GM, Mohan KM, Holte SE, De Vange SM, Pawluk DM, Melvin AJ, Lewis PF, Heath LM, Beck IA, Mahalanabis M, Naugler WE, Tobin NH, Mullins JI. Multiple viral genetic analyses detect low-level human immunodeficiency virus type 1 replication during effective highly active antiretroviral therapy. J Virol. 2003;77:5721–5730. doi: 10.1128/JVI.77.10.5721-5730.2003. - DOI - PMC - PubMed
-
- Tobin NH, Learn GH, Holte SE, Wang Y, Melvin AJ, McKernan JL, Pawluk DM, Mohan KM, Lewis PF, Mullins JI, Frenkel LM. Evidence that low-level viremias during effective highly active antiretroviral therapy result from two processes: expression of archival virus and replication of virus. J Virol. 2005;79:9625–9634. doi: 10.1128/JVI.79.15.9625-9634.2005. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

