Transmission of avian influenza virus (H3N2) to dogs
- PMID: 18439355
- PMCID: PMC2600237
- DOI: 10.3201/eid1405.071471
Transmission of avian influenza virus (H3N2) to dogs
Abstract
In South Korea, where avian influenza virus subtypes H3N2, H5N1, H6N1, and H9N2 circulate or have been detected, 3 genetically similar canine influenza virus (H3N2) strains of avian origin (A/canine/Korea/01/2007, A/canine/Korea/02/2007, and A/canine/Korea/03/2007) were isolated from dogs exhibiting severe respiratory disease. To determine whether the novel canine influenza virus of avian origin was transmitted among dogs, we experimentally infected beagles with this influenza virus (H3N2) isolate. The beagles shed virus through nasal excretion, seroconverted, and became ill with severe necrotizing tracheobronchitis and bronchioalveolitis with accompanying clinical signs (e.g., high fever). Consistent with histologic observation of lung lesions, large amounts of avian influenza virus binding receptor (SAalpha 2,3-gal) were identified in canine tracheal, bronchial, and bronchiolar epithelial cells, which suggests potential for direct transmission of avian influenza virus (H3N2) from poultry to dogs. Our data provide evidence that dogs may play a role in interspecies transmission and spread of influenza virus.
Figures
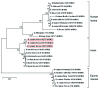
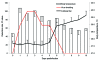


References
-
- Wright PF, Webster RG. In: Orthomyxoviruses. Philadelphia: Lippincott Williams & Wilkins; 2001. p.1533–9
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
