Loss of c/EBP-beta activity promotes the adaptive to apoptotic switch in hypoxic cortical neurons
- PMID: 18439838
- PMCID: PMC2652244
- DOI: 10.1016/j.mcn.2008.01.014
Loss of c/EBP-beta activity promotes the adaptive to apoptotic switch in hypoxic cortical neurons
Abstract
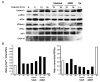
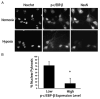
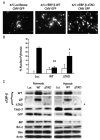
Understanding the mechanisms governing the switch between hypoxia-induced adaptive and pathological transcription may reveal novel therapeutic targets for stroke. Using an in vitro hypoxia model that temporally separates these divergent responses, we found apoptotic signaling was preceded by a decline in c/EBP-beta activity and was associated with markers of ER-stress including transient eIF2alpha phosphorylation, and the delayed induction of the bZIP proteins ATF4 and CHOP-10. Pretreatment with the eIF2alpha phosphatase inhibitor salubrinal blocked the activation of caspase-3, indicating that ER-related stress responses are integral to this transition. Delivery of either full-length, or a transcriptionally inactive form of c/EBP-beta protected cultures from hypoxic challenge, in part by inducing levels of the anti-apoptotic protein Bcl-2. These data indicate that the pathologic response in cortical neurons induced by hypoxia involves both the loss of c/EBP-beta-mediated survival signals and activation of pro-death pathways originating from the endoplasmic reticulum.
Conflict of interest statement
The authors report no commercial affiliations or conflicts of interest pertaining to this manuscript.
Figures




References
-
- Abe K, Aoki M, Kawagoe J, Yoshida T, Hattori A, Kogure K, Itoyama Y. Ischemic delayed neuronal death. A mitochondrial hypothesis. Stroke. 1995;26:1478–1489. - PubMed
-
- Ankarcrona M, Dypbukt JM, Bonfoco E, Zhivotovsky B, Orrenius S, Lipton SA, Nicotera P. Glutamate-induced neuronal death: a succession of necrosis or apoptosis depending on mitochondrial function. Neuron. 1995;15:961–973. - PubMed
-
- Banasiak KJ, Haddad GG. Hypoxia-induced apoptosis: effect of hypoxic severity and role of p53 in neuronal cell death. Brain Res. 1998;797:295–304. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

