LYRIC/AEG-1 overexpression modulates BCCIPalpha protein levels in prostate tumor cells
- PMID: 18440304
- PMCID: PMC2573900
- DOI: 10.1016/j.bbrc.2008.04.084
LYRIC/AEG-1 overexpression modulates BCCIPalpha protein levels in prostate tumor cells
Abstract
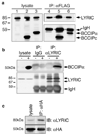
LYRIC/AEG-1 is a unique protein that has been shown to promote tumor cell migration and invasion through activation of the transcription factor NF-kappaB. We performed yeast two-hybrid screening to detect LYRIC/AEG-1 associated proteins, and identified BCCIP, a CDKN1A and BRCA2-associated protein involved in cell cycle regulation and DNA repair. Here, we demonstrate association between LYRIC/AEG-1 and BCCIP in mammalian cells, and define the region of interaction. Co-expression of the two proteins resulted in decreased levels of BCCIPalpha, an effect partially abrogated by proteasome inhibition. A truncated LYRIC/AEG-1 construct lacking the interaction region did not alter BCCIPalpha protein levels. Coincidentally, it was observed that overexpression of BCCIPalpha in DU145 prostate tumor cells induced an apparent neuroendocrine differentiation. In summary, our data suggest LYRIC/AEG-1 is a negative regulator of BCCIPalpha, promoting proteasomal degradation either through direct interaction, or potentially through an indirect mechanism involving downstream effects of the NF-kappaB signaling pathway.
Figures



References
-
- Kang DC, Su ZZ, Sarkar D, Emdad L, Volsky DJ, Fisher PB. Cloning and characterization of HIV-1-inducible astrocyte elevated gene-1, AEG-1. Gene. 2005;353:8–15. - PubMed
-
- Brown DM, Ruoslahti E. Metadherin, a cell surface protein in breast tumors that mediates lung metastasis. Cancer Cell. 2004;5:365–374. - PubMed
-
- Kikuno N, Shiina H, Urakami S, Kawamoto K, Hirata H, Tanaka Y, Place RF, Pookot D, Majid S, Igawa M, Dahiya R. Knockdown of astrocyte-elevated gene-1 inhibits prostate cancer progression through upregulation of FOXO3a activity. Oncogene. 2007;26:7647–7655. - PubMed
-
- van 't Veer LJ, Dai H, van de Vijver MJ, He YD, Hart AA, Mao M, Peterse HL, van der Kooy K, Marton MJ, Witteveen AT, Schreiber GJ, Kerkhoven RM, Roberts C, Linsley PS, Bernards R, Friend SH. Gene expression profiling predicts clinical outcome of breast cancer. Nature. 2002;415:530–536. - PubMed
-
- Emdad L, Sarkar D, Su ZZ, Randolph A, Boukerche H, Valerie K, Fisher PB. Activation of the nuclear factor kappaB pathway by astrocyte elevated gene-1: implications for tumor progression and metastasis. Cancer Res. 2006;66:1509–1516. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

