Mild traumatic brain injury to the infant mouse causes robust white matter axonal degeneration which precedes apoptotic death of cortical and thalamic neurons
- PMID: 18440507
- PMCID: PMC2486437
- DOI: 10.1016/j.expneurol.2008.03.012
Mild traumatic brain injury to the infant mouse causes robust white matter axonal degeneration which precedes apoptotic death of cortical and thalamic neurons
Abstract
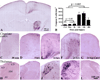
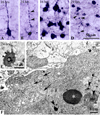
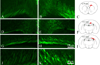
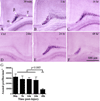
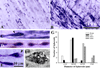
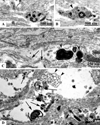
The immature brain in the first several years of childhood is very vulnerable to trauma. Traumatic brain injury (TBI) during this critical period often leads to neuropathological and cognitive impairment. Previous experimental studies in rodent models of infant TBI were mostly concentrated on neuronal degeneration, while axonal injury and its relationship to cell death have attracted much less attention. To address this, we developed a closed controlled head injury model in infant (P7) mice and characterized the temporospatial pattern of axonal degeneration and neuronal cell death in the brain following mild injury. Using amyloid precursor protein (APP) as marker of axonal injury we found that mild head trauma causes robust axonal degeneration in the cingulum/external capsule as early as 30 min post-impact. These levels of axonal injury persisted throughout a 24 h period, but significantly declined by 48 h. During the first 24 h injured axons underwent significant and rapid pathomorphological changes. Initial small axonal swellings evolved into larger spheroids and club-like swellings indicating the early disconnection of axons. Ultrastructural analysis revealed compaction of organelles, axolemmal and cytoskeletal defects. Axonal degeneration was followed by profound apoptotic cell death in the posterior cingulate and retrosplenial cortex and anterior thalamus which peaked between 16 and 24 h post-injury. At early stages post-injury no evidence of excitotoxic neuronal death at the impact site was found. At 48 h apoptotic cell death was reduced and paralleled with the reduction in the number of APP-labeled axonal profiles. Our data suggest that early degenerative response to injury in axons of the cingulum and external capsule may cause disconnection between cortical and thalamic neurons, and lead to their delayed apoptotic death.
Figures

References
-
- Adelson PD, Robichaud P, Hamilton RL, Kochanek PM. A model of diffuse traumatic brain injury in the immature rat. J Neurosurg. 1996;85:877–884. - PubMed
-
- Adelson PD, Jenkins LW, Hamilton RL, Robichaud P, Tran MP, Kochanek PM. Histopathologic response of the immature rat to diffuse traumatic brain injury. J Neurotrauma. 2001;18:967–976. - PubMed
-
- Anderson V, Catroppa C, Morse S, Haritou F, Rosenfeld J. Functional plasticity or vulnerability after early brain injury? Pediatrics. 2005;116:1374–1382. - PubMed
-
- Bailey CD, Johnson GV. Developmental regulation of tissue transglutaminase in the mouse forebrain. J Neurochem. 2004;91:1369–1379. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

