A G-tract element in apoptotic agents-induced alternative splicing
- PMID: 18440980
- PMCID: PMC2425498
- DOI: 10.1093/nar/gkn207
A G-tract element in apoptotic agents-induced alternative splicing
Abstract
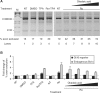
Alternative splicing of a single pre-mRNA transcript can produce protein isoforms that promote either cell growth or death. Here we show that Ro-31-8220 (Ro), an apoptotic agent that inhibits protein kinase C and activates the c-Jun N terminal kinase, decreased the proportion of the cell growth-promoting Bcl-xL splice variant. Targeted mutagenesis analyses narrowed down a critical sequence to a 16-nt G-tract element (Gt16). Transferring this element to a heterologous gene conferred Ro response on an otherwise constitutive exon. The Ro effect was reduced by okadaic acid, an inhibitor of protein phosphatases PP1 and PP2A, in a concentration-dependent manner. Search in the human genome followed by RT-PCR identified a group of genes that contain similar exonic G-tract elements and are responsive to Ro. Moreover, the Gt16 element also mediates the regulation of alternative splicing by other cell apoptosis-inducers particularly retinoic acid. Therefore, the G-tract element likely plays a role in the apoptotic agents-induced alternative splicing of a group of genes. The functions of these genes imply that this regulation will have impact on cell growth/death.
Figures
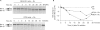

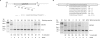
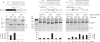
Similar articles
-
Identification of a novel cis-element that regulates alternative splicing of Bcl-x pre-mRNA.Biochem Biophys Res Commun. 2012 Apr 6;420(2):467-72. doi: 10.1016/j.bbrc.2012.03.029. Epub 2012 Mar 13. Biochem Biophys Res Commun. 2012. PMID: 22440396
-
Emetine regulates the alternative splicing of Bcl-x through a protein phosphatase 1-dependent mechanism.Chem Biol. 2007 Dec;14(12):1386-92. doi: 10.1016/j.chembiol.2007.11.004. Chem Biol. 2007. PMID: 18096507 Free PMC article.
-
De novo ceramide regulates the alternative splicing of caspase 9 and Bcl-x in A549 lung adenocarcinoma cells. Dependence on protein phosphatase-1.J Biol Chem. 2002 Apr 12;277(15):12587-95. doi: 10.1074/jbc.M112010200. Epub 2002 Jan 18. J Biol Chem. 2002. PMID: 11801602
-
Splice variants in apoptotic pathway.Exp Oncol. 2012 Oct;34(3):212-7. Exp Oncol. 2012. PMID: 23070006 Review.
-
Aspects of splice site selection in constitutive and alternative pre-mRNA splicing.Gene Expr. 1995;4(3):177-82. Gene Expr. 1995. PMID: 7734950 Free PMC article. Review.
Cited by
-
Differential evolution of signal-responsive RNA elements and upstream factors that control alternative splicing.Cell Mol Life Sci. 2014 Nov;71(22):4347-60. doi: 10.1007/s00018-014-1688-y. Epub 2014 Jul 27. Cell Mol Life Sci. 2014. PMID: 25064062 Free PMC article. Review.
-
Evolutionary emergence of a novel splice variant with an opposite effect on the cell cycle.Mol Cell Biol. 2015 Jun;35(12):2203-14. doi: 10.1128/MCB.00190-15. Epub 2015 Apr 13. Mol Cell Biol. 2015. PMID: 25870105 Free PMC article.
-
Regulating Divergent Transcriptomes through mRNA Splicing and Its Modulation Using Various Small Compounds.Int J Mol Sci. 2020 Mar 16;21(6):2026. doi: 10.3390/ijms21062026. Int J Mol Sci. 2020. PMID: 32188117 Free PMC article. Review.
-
The heterogeneous nuclear ribonucleoprotein L is an essential component in the Ca2+/calmodulin-dependent protein kinase IV-regulated alternative splicing through cytidine-adenosine repeats.J Biol Chem. 2009 Jan 16;284(3):1505-13. doi: 10.1074/jbc.M805113200. Epub 2008 Nov 18. J Biol Chem. 2009. PMID: 19017650 Free PMC article.
-
Control of alternative splicing by forskolin through hnRNP K during neuronal differentiation.Nucleic Acids Res. 2012 Sep;40(16):8059-71. doi: 10.1093/nar/gks504. Epub 2012 Jun 8. Nucleic Acids Res. 2012. PMID: 22684629 Free PMC article.
References
-
- Maniatis T, Tasic B. Alternative pre-mRNA splicing and proteome expansion in metazoans. Nature. 2002;418:236–243. - PubMed
-
- Black DL. Protein diversity from alternative splicing: a challenge for bioinformatics and post-genome biology. Cell. 2000;103:367–370. - PubMed
-
- Black DL. Mechanisms of alternative pre-messenger RNA splicing. Annu. Rev. Biochem. 2003;27:27. - PubMed
-
- Graveley BR. Alternative splicing: increasing diversity in the proteomic world. Trends Genet. 2001;17:100–107. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

