Cooperativity and specificity in enzyme kinetics: a single-molecule time-based perspective
- PMID: 18441030
- PMCID: PMC2426636
- DOI: 10.1529/biophysj.108.131771
Cooperativity and specificity in enzyme kinetics: a single-molecule time-based perspective
Abstract
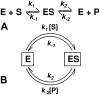
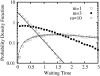
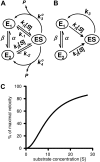
An alternative theoretical approach to enzyme kinetics that is particularly applicable to single-molecule enzymology is presented. The theory, originated by Van Slyke and Cullen in 1914, develops enzyme kinetics from a "time perspective" rather than the traditional "rate perspective" and emphasizes the nonequilibrium steady-state nature of enzymatic reactions and the significance of small copy numbers of enzyme molecules in living cells. Sigmoidal cooperative substrate binding to slowly fluctuating, monomeric enzymes is shown to arise from association pathways with very small probability but extremely long passage time, which would be disregarded in the traditional rate perspective: A single enzyme stochastically takes alternative pathways in serial order rather than different pathways in parallel. The theory unifies dynamic cooperativity and Hopfield-Ninio's kinetic proofreading mechanism for specificity amplification.
Figures

References
-
- Beard, D. A., and H. Qian. 2008. Chemical biophysics: quantitative analysis of cellular systems. In Cambridge Texts in Biomedical Engineering. Cambridge University Press, New York.
-
- Cornish-Bowden, A. 2004. Fundamentals of Enzyme Kinetics. Portland Press, London, UK.
-
- English, B. P., W. Min, A. M. van Oijen, K. T. Lee, G. Luo, H. Sun, B. J. Cherayil, S. C. Kou, and X. S. Xie. 2006. Ever-fluctuating single enzyme molecules: Michaelis-Menten equation revisited. Nat. Chem. Biol. 2:87–94. - PubMed
-
- Qian, H. 2002. Equations for stochastic macromolecular mechanics of single proteins: equilibrium fluctuations, transient kinetics and nonequilibrium steady-state. J. Phys. Chem. B. 106:2065–2073.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

