Diffusive coupling and network periodicity: a computational study
- PMID: 18441034
- PMCID: PMC2479614
- DOI: 10.1529/biophysj.108.129239
Diffusive coupling and network periodicity: a computational study
Abstract
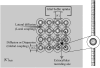
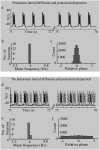
Diffusive coupling (nearest-neighbor coupling) is the most common type of coupling present in many systems. Previous experimental and theoretical studies have shown that potassium lateral diffusion coupling (i.e., diffusive coupling) can be responsible for synchronization of neuronal activity. Recent in vivo experiments carried out with anesthetized rat hippocampus suggested that the extracellular potassium could play an important role in the generation of a novel type of epileptiform nonsynaptic activity. Yet, the role of potassium in the generation of seizures remains controversial. We tested the hypothesis that potassium lateral diffusion coupling is responsible for the coupling mechanisms for network periodicity in a nonsynaptic model of epilepsy in vivo using a CA1 pyramidal neuron network model The simulation results show that 1), potassium lateral diffusion coupling is crucial for establishing epileptiform activity similar to that generated experimentally; and 2), there exists a scaling relation between the critical coupling strength and the number of cells in the network. The results not only agree with the theoretical prediction, but strongly suggest that potassium lateral diffusion coupling, a physiological realization of the concept of diffusive coupling, can play an important role in entraining periodicity in a nonsynaptic neural network.
Figures




Similar articles
-
Potassium diffusive coupling in neural networks.Philos Trans R Soc Lond B Biol Sci. 2010 Aug 12;365(1551):2347-62. doi: 10.1098/rstb.2010.0050. Philos Trans R Soc Lond B Biol Sci. 2010. PMID: 20603356 Free PMC article. Review.
-
Diffusive coupling can induce synchronized periodic activity in neural networks.Annu Int Conf IEEE Eng Med Biol Soc. 2008;2008:3677-8. doi: 10.1109/IEMBS.2008.4650005. Annu Int Conf IEEE Eng Med Biol Soc. 2008. PMID: 19163508
-
Effects of extracellular potassium diffusion on electrically coupled neuron networks.Phys Rev E Stat Nonlin Soft Matter Phys. 2015 Feb;91(2):022712. doi: 10.1103/PhysRevE.91.022712. Epub 2015 Feb 23. Phys Rev E Stat Nonlin Soft Matter Phys. 2015. PMID: 25768536
-
Role of potassium lateral diffusion in non-synaptic epilepsy: a computational study.J Theor Biol. 2006 Feb 7;238(3):666-82. doi: 10.1016/j.jtbi.2005.06.015. Epub 2005 Aug 8. J Theor Biol. 2006. PMID: 16085109
-
Control theory applied to neural networks illuminates synaptic basis of interictal epileptiform activity.Adv Neurol. 1986;44:263-74. Adv Neurol. 1986. PMID: 3518346 Review.
Cited by
-
A tunable support vector machine assembly classifier for epileptic seizure detection.Expert Syst Appl. 2012 Mar 1;39(4):3925-3938. doi: 10.1016/j.eswa.2011.08.088. Epub 2011 Aug 30. Expert Syst Appl. 2012. PMID: 22563146 Free PMC article.
-
Electrodiffusive model for astrocytic and neuronal ion concentration dynamics.PLoS Comput Biol. 2013;9(12):e1003386. doi: 10.1371/journal.pcbi.1003386. Epub 2013 Dec 19. PLoS Comput Biol. 2013. PMID: 24367247 Free PMC article.
-
Enhanced multiple vibrational resonances by Na+ and K+ dynamics in a neuron model.Sci Rep. 2015 Jan 8;5:7684. doi: 10.1038/srep07684. Sci Rep. 2015. PMID: 25567752 Free PMC article.
-
Slow periodic activity in the longitudinal hippocampal slice can self-propagate non-synaptically by a mechanism consistent with ephaptic coupling.J Physiol. 2019 Jan;597(1):249-269. doi: 10.1113/JP276904. Epub 2018 Nov 10. J Physiol. 2019. PMID: 30295923 Free PMC article.
-
Potassium diffusive coupling in neural networks.Philos Trans R Soc Lond B Biol Sci. 2010 Aug 12;365(1551):2347-62. doi: 10.1098/rstb.2010.0050. Philos Trans R Soc Lond B Biol Sci. 2010. PMID: 20603356 Free PMC article. Review.
References
-
- Pikovsky, A. S., M. G. Rosenblum, and J. Kurths. 2001. Synchronization. A Universal Concept in Nonlinear Sciences. Cambridge University Press, Springer, Berlin.
-
- Okuda, K. 1993. Variety and generality of clustering in globally coupled oscillators. Physica D. 63:424–436.
-
- Bar-Eli, K. 1985. On the stability of coupled chemical oscillators. Physica D. 14:242–252.
-
- Liu, Z., Y. C. Lai, and F. C. Hoppensteadt. 2001. Phase clustering and transition to phase synchronization in a large number of coupled nonlinear oscillators. Phys. Rev. E Stat. Nonlin. Soft Matter Phys. 63:055201. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

