Utility of magnetic resonance imaging and nuclear magnetic resonance-based metabolomics for quantification of inflammatory lung injury
- PMID: 18441091
- PMCID: PMC2494785
- DOI: 10.1152/ajplung.00515.2007
Utility of magnetic resonance imaging and nuclear magnetic resonance-based metabolomics for quantification of inflammatory lung injury
Abstract
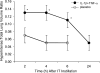
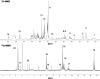
Magnetic resonance imaging (MRI) and metabolic nuclear magnetic resonance (NMR) spectroscopy are clinically available but have had little application in the quantification of experimental lung injury. There is a growing and unfulfilled need for predictive animal models that can improve our understanding of disease pathogenesis and therapeutic intervention. Integration of MRI and NMR could extend the application of experimental data into the clinical setting. This study investigated the ability of MRI and metabolic NMR to detect and quantify inflammation-mediated lung injury. Pulmonary inflammation was induced in male B6C3F1 mice by intratracheal administration of IL-1beta and TNF-alpha under isoflurane anesthesia. Mice underwent MRI at 2, 4, 6, and 24 h after dosing. At 6 and 24 h lungs were harvested for metabolic NMR analysis. Data acquired from IL-1beta+TNF-alpha-treated animals were compared with saline-treated control mice. The hyperintense-to-total lung volume (HTLV) ratio derived from MRI was higher in IL-1beta+TNF-alpha-treated mice compared with control at 2, 4, and 6 h but returned to control levels by 24 h. The ability of MRI to detect pulmonary inflammation was confirmed by the association between HTLV ratio and histological and pathological end points. Principal component analysis of NMR-detectable metabolites also showed a temporal pattern for which energy metabolism-based biomarkers were identified. These data demonstrate that both MRI and metabolic NMR have utility in the detection and quantification of inflammation-mediated lung injury. Integration of these clinically available techniques into experimental models of lung injury could improve the translation of basic science knowledge and information to the clinic.
Figures


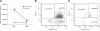

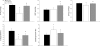
References
-
- Beckmann N, Cannet C, Karmouty-Quintana H, Tigani B, Zurbruegg S, Ble FX, Cremillieux Y, Trifilieff A. Lung MRI for experimental drug research. Eur J Radiol 64: 381–396, 2007. - PubMed
-
- Beckmann N, Tigani B, Ekatodramis D, Borer R, Mazzoni L, Fozard JR. Pulmonary edema induced by allergen challenge in the rat: noninvasive assessment by magnetic resonance imaging. Magn Reson Med 45: 88–95, 2001. - PubMed
-
- Brealey D, Brand M, Hargreaves I, Heales S, Land J, Smolenski R, Davies NA, Cooper CE, Singer M. Association between mitochondrial dysfunction and severity and outcome of septic shock. Lancet 360: 219–223, 2002. - PubMed
-
- Caruthers SD, Paschal CB, Pou NA, Roselli RJ, Harris TR. Regional measurements of pulmonary edema by using magnetic resonance imaging. J Appl Physiol 84: 2143–2153, 1998. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

