Modulation of antioxidant defense in Aspergillus parasiticus is involved in aflatoxin biosynthesis: a role for the ApyapA gene
- PMID: 18441122
- PMCID: PMC2446656
- DOI: 10.1128/EC.00228-07
Modulation of antioxidant defense in Aspergillus parasiticus is involved in aflatoxin biosynthesis: a role for the ApyapA gene
Abstract
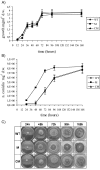
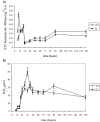
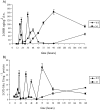
Oxidative stress is recognized as a trigger of different metabolic events in all organisms. Various factors correlated with oxidation, such as the beta-oxidation of fatty acids and their enzymatic or nonenzymatic by-products (e.g., precocious sexual inducer factors and lipoperoxides) have been shown to be involved in aflatoxin formation. In the present study, we found that increased levels of reactive oxygen species (ROS) were correlated with increased levels of aflatoxin biosynthesis in Aspergillus parasiticus. To better understand the role of ROS formation in toxin production, we generated a mutant (Delta ApyapA) having the ApyapA gene deleted, given that ApyapA orthologs have been shown to be part of the antioxidant response in other fungi. Compared to the wild type, the mutant showed an increased susceptibility to extracellular oxidants, as well as precocious ROS formation and aflatoxin biosynthesis. Genetic complementation of the Delta ApyapA mutant restored the timing and quantity of toxin biosynthesis to the levels found in the wild type. The presence of putative AP1 (ApYapA orthologue) binding sites in the promoter region of the regulatory gene aflR further supports the finding that ApYapA plays a role in the regulation of aflatoxin biosynthesis. Overall, our results show that the lack of ApyapA leads to an increase in oxidative stress, premature conidiogenesis, and aflatoxin biosynthesis.
Figures




References
-
- Aguirre, J., M. Rios-Momberg, D. Hewitt, and W. Hansberg. 2005. Reactive oxygen species and development in microbial eukaryotes. Trends Microbiol. 13111-118. - PubMed
-
- Aguirre, J., W. Hansberg, and R. Navarro. 2006. Fungal responses to reactive oxygen species. Med. Mycol. 44(Suppl.)101-107. - PubMed
-
- Bokoch, G. M., and U. G. Knaus. 2003. NADPH oxidase: not just for leukocytes anymore! Trends Biochem. Sci. 28502-508. - PubMed
-
- Bolwell, G. P., L. V. Bindschedler, K. A. Blee, V. S. Butt, D. R. Davies, S. L. Gardner, C. Gerrish, and F. Minibayeva. 2002. The apoplastic oxidative burst in response to biotic stress in plants: a three-component system. J. Exp. Bot. 531367-1376. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

