Functional characterization of a redundant Plasmodium TRAP family invasin, TRAP-like protein, by aldolase binding and a genetic complementation test
- PMID: 18441124
- PMCID: PMC2446664
- DOI: 10.1128/EC.00089-08
Functional characterization of a redundant Plasmodium TRAP family invasin, TRAP-like protein, by aldolase binding and a genetic complementation test
Abstract
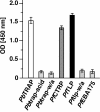
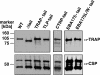
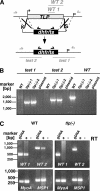
Efficient and specific host cell entry is of exquisite importance for intracellular pathogens. Parasites of the phylum Apicomplexa are highly motile and actively enter host cells. These functions are mediated by type I transmembrane invasins of the TRAP family that link an extracellular recognition event to the parasite actin-myosin motor machinery. We systematically tested potential parasite invasins for binding to the actin bridging molecule aldolase and complementation of the vital cytoplasmic domain of the sporozoite invasin TRAP. We show that the ookinete invasin CTRP and a novel, structurally related protein, termed TRAP-like protein (TLP), are functional members of the TRAP family. Although TLP is expressed in invasive stages, targeted gene disruption revealed a nonvital role during life cycle progression. This is the first genetic analysis of TLP, encoding a redundant TRAP family invasin, in the malaria parasite.
Figures



References
-
- Baum, J., A. T. Papenfuss, B. Baum, T. P. Speed, and A. F. Cowman. 2006. Regulation of apicomplexan actin-based motility. Nat. Rev. Microbiol. 4621-628. - PubMed
-
- Baum, J., D. Richard, J. Healer, M. Rug, Z. Krnajski, T.-W. Gilberger, J. L. Green, A. A. Holder, and A. F. Cowman. 2006. A conserved molecular motor drives cell invasion and gliding motility across malaria life cycle stages and other apicomplexan parasites. J. Biol. Chem. 2815197-5208. - PubMed
-
- Bergman, L. W., K. Kaiser, H. Fujioka, I. Coppens, T. M. Daly, S. Fox, K. Matuschewski, V. Nussenzweig, and S. H. I. Kappe. 2003. Myosin A tail domain interacting protein (MTIP) localizes to the inner membrane complex of Plasmodium sporozoites. J. Cell Sci. 11639-49. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

