Intravitreal properties of porous silicon photonic crystals: a potential self-reporting intraocular drug-delivery vehicle
- PMID: 18441177
- PMCID: PMC2666262
- DOI: 10.1136/bjo.2007.133587
Intravitreal properties of porous silicon photonic crystals: a potential self-reporting intraocular drug-delivery vehicle
Abstract
Aim: To determine the suitability of porous silicon photonic crystals for intraocular drug-delivery.
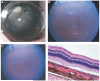
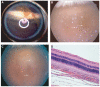
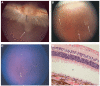
Methods: A rugate structure was electrochemically etched into a highly doped p-type silicon substrate to create a porous silicon film that was subsequently removed and ultrasonically fractured into particles. To stabilise the particles in aqueous media, the silicon particles were modified by surface alkylation (using thermal hydrosilylation) or by thermal oxidation. Unmodified particles, hydrosilylated particles and oxidised particles were injected into rabbit vitreous. The stability and toxicity of each type of particle were studied by indirect ophthalmoscopy, biomicroscopy, tonometry, electroretinography (ERG) and histology.
Results: No toxicity was observed with any type of the particles during a period of >4 months. Surface alkylation led to dramatically increased intravitreal stability and slow degradation. The estimated vitreous half-life increased from 1 week (fresh particles) to 5 weeks (oxidised particles) and to 16 weeks (hydrosilylated particles).
Conclusion: The porous silicon photonic crystals showed good biocompatibility and may be used as an intraocular drug-delivery system. The intravitreal injectable porous silicon photonic crystals may be engineered to host a variety of therapeutics and achieve controlled drug release over long periods of time to treat chronic vitreoretinal diseases.
Figures



Similar articles
-
Oxidized porous silicon particles covalently grafted with daunorubicin as a sustained intraocular drug delivery system.Invest Ophthalmol Vis Sci. 2013 Feb 1;54(2):1268-79. doi: 10.1167/iovs.12-11172. Invest Ophthalmol Vis Sci. 2013. PMID: 23322571 Free PMC article.
-
Hydrosilylated porous silicon particles function as an intravitreal drug delivery system for daunorubicin.J Ocul Pharmacol Ther. 2013 Jun;29(5):493-500. doi: 10.1089/jop.2012.0205. Epub 2013 Feb 28. J Ocul Pharmacol Ther. 2013. PMID: 23448595 Free PMC article.
-
Controlled Release of Dexamethasone From an Intravitreal Delivery System Using Porous Silicon Dioxide.Invest Ophthalmol Vis Sci. 2016 Feb;57(2):557-66. doi: 10.1167/iovs.15-18559. Invest Ophthalmol Vis Sci. 2016. PMID: 26882530 Free PMC article.
-
Porous silicon in drug delivery devices and materials.Adv Drug Deliv Rev. 2008 Aug 17;60(11):1266-1277. doi: 10.1016/j.addr.2008.03.017. Epub 2008 Apr 10. Adv Drug Deliv Rev. 2008. PMID: 18508154 Free PMC article. Review.
-
Drug delivery via porous silicon: a focused patent review.Pharm Pat Anal. 2017 Mar;6(2):77-85. doi: 10.4155/ppa-2016-0042. Epub 2017 Mar 1. Pharm Pat Anal. 2017. PMID: 28248125 Review.
Cited by
-
Oxidized porous silicon particles covalently grafted with daunorubicin as a sustained intraocular drug delivery system.Invest Ophthalmol Vis Sci. 2013 Feb 1;54(2):1268-79. doi: 10.1167/iovs.12-11172. Invest Ophthalmol Vis Sci. 2013. PMID: 23322571 Free PMC article.
-
Sustained Release of a Monoclonal Antibody from Electrochemically Prepared Mesoporous Silicon Oxide.Adv Funct Mater. 2010 Sep 8;20(23):4168-4174. doi: 10.1002/adfm.201000907. Adv Funct Mater. 2010. PMID: 21274422 Free PMC article.
-
Intravitreal safety profiles of sol-gel mesoporous silica microparticles and the degradation product (Si(OH)4).Drug Deliv. 2020 Dec;27(1):703-711. doi: 10.1080/10717544.2020.1760401. Drug Deliv. 2020. PMID: 32393079 Free PMC article.
-
Intraocular pressure changes: an important determinant of the biocompatibility of intravitreous implants.PLoS One. 2011;6(12):e28720. doi: 10.1371/journal.pone.0028720. Epub 2011 Dec 14. PLoS One. 2011. PMID: 22194895 Free PMC article.
-
Ocular silicon distribution and clearance following intravitreal injection of porous silicon microparticles.Exp Eye Res. 2013 Nov;116:161-8. doi: 10.1016/j.exer.2013.09.001. Epub 2013 Sep 10. Exp Eye Res. 2013. PMID: 24036388 Free PMC article.
References
-
- D’Amico DJ, Bird AC. VEGF Inhibition study in ocular neovascularization-1 (VISION-1): safety evaluation from the pivotal Macugen™ (pegaptanib sodium) clinical trials [E-abstract] Invest Ophthalmol Vis Sci. 2004;45:2363.
-
- Martin DF, Ferris FL, Parks DJ, et al. Ganciclovir implant exchange. Timing, surgical procedure, and complications. Arch Ophthalmol. 1997;115:1389–94. - PubMed
-
- Cheng L, Hostetler KY, Chaidhawangul S, et al. Treatment or prevention of herpes simplex virus retinitis with intravitreally injectable crystalline 1-O-hexadecylpropanediol-3-phospho-ganciclovir. Invest Ophthalmol Vis Sci. 2002;43:515–21. - PubMed
-
- Sailor MJ, Trogler WC, Content S, et al. In: Carapezza EM, Law DB, Stalker KT, editors. Detection of DNT, TNT, HF and nerve agents using photoluminescence and interferometry from a porous silicon chip; SPIE Meeting on Unattended Ground Sensor Technologies and Applications; Orlando, FL: SPIE. 2000.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
