Critical role of extracellular heat shock cognate protein 70 in the myocardial inflammatory response and cardiac dysfunction after global ischemia-reperfusion
- PMID: 18441202
- PMCID: PMC3137641
- DOI: 10.1152/ajpheart.00299.2008
Critical role of extracellular heat shock cognate protein 70 in the myocardial inflammatory response and cardiac dysfunction after global ischemia-reperfusion
Abstract
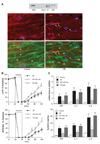
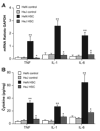
Previous studies showed that Toll-like receptor 4 (TLR4) modulates the myocardial inflammatory response to ischemia-reperfusion injury, and we recently found that cytokines link TLR4 to postischemic cardiac dysfunction. Although TLR4 can be activated in cultured cells by endogenous agents including heat shock protein 70, how it is activated during myocardial ischemia-reperfusion is unknown. In the present study, we examined 1) whether heat shock cognate protein 70 (HSC70), which is constitutively expressed in the myocardium, is released during ischemia-reperfusion; 2) whether extracellular HSC70 induces the myocardial inflammatory response and modulates cardiac function; and 3) whether HSC70 exerts these effects via TLR4. We subjected isolated mouse hearts to global ischemia-reperfusion via the Langendorff technique. Immunoblotting and immunostaining detected the release of HSC70 from the myocardium during reperfusion. Treatment with an antibody specific to HSC70 suppressed myocardial cytokine expression and improved cardiac functional recovery after ischemia-reperfusion. Recombinant HSC70 induced NF-kappaB activation and cytokine expression and depressed myocardial contractility in a TLR4-dependent manner. These effects required the substrate-binding domain of HSC70. Fluorescence resonance energy transfer analysis of isolated macrophages demonstrated that extracellular HSC70 interacts with TLR4. Therefore, this study demonstrates for the first time that 1) the myocardium releases HSC70 during ischemia-reperfusion, 2) extracellular HSC70 contributes to the postischemic myocardial inflammatory response and to cardiac dysfunction, 3) HSC70 exerts these effects through a TLR4-dependent mechanism, and 4) the substrate-binding domain of HSC70 is required to induce these effects. Thus extracellular HSC70 plays a critical role in regulating the myocardial innate immune response and cardiac function after ischemia-reperfusion.
Figures




Similar articles
-
Myocardial TLR4 is a determinant of neutrophil infiltration after global myocardial ischemia: mediating KC and MCP-1 expression induced by extracellular HSC70.Am J Physiol Heart Circ Physiol. 2009 Jul;297(1):H21-8. doi: 10.1152/ajpheart.00292.2009. Epub 2009 May 15. Am J Physiol Heart Circ Physiol. 2009. PMID: 19448144 Free PMC article.
-
Human myocardium releases heat shock protein 27 (HSP27) after global ischemia: the proinflammatory effect of extracellular HSP27 through toll-like receptor (TLR)-2 and TLR4.Mol Med. 2014 Jun 9;20(1):280-9. doi: 10.2119/molmed.2014.00058. Mol Med. 2014. PMID: 24918749 Free PMC article.
-
Cytokines link Toll-like receptor 4 signaling to cardiac dysfunction after global myocardial ischemia.Ann Thorac Surg. 2008 May;85(5):1678-85. doi: 10.1016/j.athoracsur.2008.01.043. Ann Thorac Surg. 2008. PMID: 18442564 Free PMC article.
-
The role of Toll-like receptor 4 (TLR4) in cardiac ischaemic-reperfusion injury, cardioprotection and preconditioning.Clin Exp Pharmacol Physiol. 2016 Sep;43(9):864-71. doi: 10.1111/1440-1681.12602. Clin Exp Pharmacol Physiol. 2016. PMID: 27249055 Review.
-
Innate immunity and cardiomyocytes in ischemic heart disease.Life Sci. 2014 Mar 28;100(1):1-8. doi: 10.1016/j.lfs.2014.01.062. Epub 2014 Jan 28. Life Sci. 2014. PMID: 24486305 Free PMC article. Review.
Cited by
-
Isolated hearts treated with skeletal muscle homogenates exhibit altered function.Cell Stress Chaperones. 2013 Sep;18(5):675-81. doi: 10.1007/s12192-013-0418-y. Epub 2013 Mar 23. Cell Stress Chaperones. 2013. PMID: 23526129 Free PMC article.
-
Attenuated recovery of contractile function in aging hearts following global ischemia/reperfusion: Role of extracellular HSP27 and TLR4.Mol Med. 2017 Jan;23:863-872. doi: 10.2119/molmed.2016.00204. Epub 2016 Dec 19. Mol Med. 2017. PMID: 28079228 Free PMC article.
-
Toll-like receptor 4 signaling activates ERG function in prostate cancer and provides a therapeutic target.NAR Cancer. 2021 Jan 27;3(1):zcaa046. doi: 10.1093/narcan/zcaa046. eCollection 2021 Mar. NAR Cancer. 2021. PMID: 33554122 Free PMC article.
-
Toll-like receptors and myocardial inflammation.Int J Inflam. 2011;2011:170352. doi: 10.4061/2011/170352. Epub 2011 Sep 29. Int J Inflam. 2011. PMID: 21977329 Free PMC article.
-
Activation of immune signals during organ transplantation.Signal Transduct Target Ther. 2023 Mar 11;8(1):110. doi: 10.1038/s41392-023-01377-9. Signal Transduct Target Ther. 2023. PMID: 36906586 Free PMC article. Review.
References
-
- Asea A, Rehli M, Kabingu E, Boch JA, Bare O, Auron PE, Stevenson MA, Calderwood SK. Novel signal transduction pathway utilized by extracellular HSP70: role of toll-like receptor (TLR) 2 and TLR4. J Biol Chem. 2002;277:15028–15034. - PubMed
-
- Barreto A, Gonzalez JM, Kabingu E, Asea A, Fiorentino S. Stress-induced release of HSC70 from human tumors. Cell Immunol. 2003;222:97–104. - PubMed
-
- Calderwood SK, Mambula SS, Gray PJ, Jr, Theriault JR. Extracellular heat shock proteins in cell signaling. FEBS Lett. 2007;581:3689–3694. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

