Plastid transcriptomics and translatomics of tomato fruit development and chloroplast-to-chromoplast differentiation: chromoplast gene expression largely serves the production of a single protein
- PMID: 18441214
- PMCID: PMC2390737
- DOI: 10.1105/tpc.107.055202
Plastid transcriptomics and translatomics of tomato fruit development and chloroplast-to-chromoplast differentiation: chromoplast gene expression largely serves the production of a single protein
Abstract
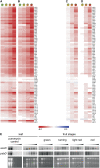
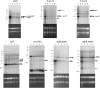
Plastid genes are expressed at high levels in photosynthetically active chloroplasts but are generally believed to be drastically downregulated in nongreen plastids. The genome-wide changes in the expression patterns of plastid genes during the development of nongreen plastid types as well as the contributions of transcriptional versus translational regulation are largely unknown. We report here a systematic transcriptomics and translatomics analysis of the tomato (Solanum lycopersicum) plastid genome during fruit development and chloroplast-to-chromoplast conversion. At the level of RNA accumulation, most but not all plastid genes are strongly downregulated in fruits compared with leaves. By contrast, chloroplast-to-chromoplast differentiation during fruit ripening is surprisingly not accompanied by large changes in plastid RNA accumulation. However, most plastid genes are translationally downregulated during chromoplast development. Both transcriptional and translational downregulation are more pronounced for photosynthesis-related genes than for genes involved in gene expression, indicating that some low-level plastid gene expression must be sustained in chromoplasts. High-level expression during chromoplast development identifies accD, the only plastid-encoded gene involved in fatty acid biosynthesis, as the target gene for which gene expression activity in chromoplasts is maintained. In addition, we have determined the developmental patterns of plastid RNA polymerase activities, intron splicing, and RNA editing and report specific developmental changes in the splicing and editing patterns of plastid transcripts.
Figures





Similar articles
-
Genome-wide analysis of plastid gene expression in potato leaf chloroplasts and tuber amyloplasts: transcriptional and posttranscriptional control.Plant Physiol. 2009 Aug;150(4):2030-44. doi: 10.1104/pp.109.140483. Epub 2009 Jun 3. Plant Physiol. 2009. PMID: 19493969 Free PMC article.
-
Comprehensive analysis of plastid gene expression during fruit development and ripening of kiwifruit.Plant Cell Rep. 2022 Apr;41(4):1103-1114. doi: 10.1007/s00299-022-02840-7. Epub 2022 Feb 28. Plant Cell Rep. 2022. PMID: 35226116
-
Design of chimeric expression elements that confer high-level gene activity in chromoplasts.Plant J. 2013 Feb;73(3):368-79. doi: 10.1111/tpj.12031. Epub 2012 Dec 10. Plant J. 2013. PMID: 23004223
-
Differentiation of chromoplasts and other plastids in plants.Plant Cell Rep. 2019 Jul;38(7):803-818. doi: 10.1007/s00299-019-02420-2. Epub 2019 May 11. Plant Cell Rep. 2019. PMID: 31079194 Free PMC article. Review.
-
Chromoplast differentiation: current status and perspectives.Plant Cell Physiol. 2010 Oct;51(10):1601-11. doi: 10.1093/pcp/pcq136. Epub 2010 Aug 27. Plant Cell Physiol. 2010. PMID: 20801922 Review.
Cited by
-
Silencing of the nuclear RPS10 gene encoding mitochondrial ribosomal protein alters translation in arabidopsis mitochondria.Plant Cell. 2013 May;25(5):1855-67. doi: 10.1105/tpc.113.111294. Epub 2013 May 30. Plant Cell. 2013. PMID: 23723321 Free PMC article.
-
Effects of inefficient transcription termination of rbcL on the expression of accD in plastids of Arabidopsis thaliana.Photosynth Res. 2015 Dec;126(2-3):323-30. doi: 10.1007/s11120-015-0159-0. Epub 2015 May 24. Photosynth Res. 2015. PMID: 26003009
-
Identification and characterization of high molecular weight complexes formed by matrix AAA proteases and prohibitins in mitochondria of Arabidopsis thaliana.J Biol Chem. 2010 Apr 23;285(17):12512-21. doi: 10.1074/jbc.M109.063644. Epub 2010 Feb 19. J Biol Chem. 2010. PMID: 20172857 Free PMC article.
-
Plastid Transformation of Micro-Tom Tomato with a Hemipteran Double-Stranded RNA Results in RNA Interference in Multiple Insect Species.Int J Mol Sci. 2022 Apr 1;23(7):3918. doi: 10.3390/ijms23073918. Int J Mol Sci. 2022. PMID: 35409279 Free PMC article.
-
A comparative genomics approach identifies a PPR-DYW protein that is essential for C-to-U editing of the Arabidopsis chloroplast accD transcript.RNA. 2009 Jun;15(6):1142-53. doi: 10.1261/rna.1533909. Epub 2009 Apr 24. RNA. 2009. PMID: 19395655 Free PMC article.
References
-
- Barkan, A. (1998). Approaches to investigating nuclear genes that function in chloroplast biogenesis in land plants. Methods Enzymol. 297 38–57.
-
- Barkan, A., and Goldschmidt-Clermont, M. (2000). Participation of nuclear genes in chloroplast gene expression. Biochimie 82 559–572. - PubMed
-
- Bathgate, B., Purton, M.E., Grierson, D., and Goodenough, P.W. (1985). Plastic changes during the conversion of chloroplasts to chromoplasts in ripening tomatoes. Planta 165 197–204. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

