Distal airways in mice exposed to cigarette smoke: Nrf2-regulated genes are increased in Clara cells
- PMID: 18441282
- PMCID: PMC2551701
- DOI: 10.1165/rcmb.2007-0295OC
Distal airways in mice exposed to cigarette smoke: Nrf2-regulated genes are increased in Clara cells
Abstract
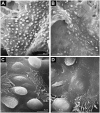
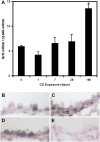
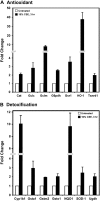
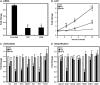
Cigarette smoke (CS) is the main risk factor for chronic obstructive pulmonary disease (COPD). Terminal bronchioles are critical zones in the pathophysiology of COPD, but little is known about the cellular and molecular changes that occur in cells lining terminal bronchioles in response to CS. We subjected C57BL/6 mice to CS (6 d/wk, up to 6 mo), looked for morphologic changes lining the terminal bronchioles, and used laser capture microdissection to selectively isolate cells in terminal bronchioles to study gene expression. Morphologic and immunohistochemical analyses showed that Clara cell predominance remained despite 6 months of CS exposure. Since Clara cells have a role in protection against oxidative stress, we focused on the expression of antioxidant/detoxification genes using microarray analysis. Of the 35 antioxidant/detoxification genes with at least 2.5-fold increased expression in response to 6 months of CS exposure, 21 were NF-E2-related factor 2 (Nrf2)-regulated genes. Among these were cytochrome P450 1b1, glutathione reductase, thioredoxin reductase, and members of the glutathione S-transferase family, as well as Nrf2 itself. In vitro studies using immortalized murine Clara cells (C22) showed that CS induced the stabilization and nuclear translocation of Nrf2, which correlated with the induction of antioxidant and detoxification genes. Furthermore, decreasing Nrf2 expression by siRNA resulted in a corresponding decrease in CS-induced expression of several antioxidant and detoxification genes by C22 cells. These data suggest that the protective response by Clara cells to CS exposure is predominantly regulated by the transcription factor Nrf2.
Figures



Similar articles
-
Effects of N-acetylcysteine on Clara cells in rats with cigarette smoke exposure.Chin Med J (Engl). 2010 Feb 20;123(4):412-7. Chin Med J (Engl). 2010. PMID: 20193479
-
Down-regulated NF-E2-related factor 2 in pulmonary macrophages of aged smokers and patients with chronic obstructive pulmonary disease.Am J Respir Cell Mol Biol. 2008 Dec;39(6):673-82. doi: 10.1165/rcmb.2007-0424OC. Epub 2008 Jun 19. Am J Respir Cell Mol Biol. 2008. PMID: 18566336
-
Deletion of Keap1 in the lung attenuates acute cigarette smoke-induced oxidative stress and inflammation.Am J Respir Cell Mol Biol. 2010 May;42(5):524-36. doi: 10.1165/rcmb.2009-0054OC. Epub 2009 Jun 11. Am J Respir Cell Mol Biol. 2010. PMID: 19520915 Free PMC article.
-
Markers of anti-oxidant response in tobacco smoke exposed subjects: a data-mining review.Pulm Pharmacol Ther. 2010 Dec;23(6):482-92. doi: 10.1016/j.pupt.2010.05.006. Epub 2010 Jun 1. Pulm Pharmacol Ther. 2010. PMID: 20594977 Review.
-
NRF2 targeting: a promising therapeutic strategy in chronic obstructive pulmonary disease.Trends Mol Med. 2011 Jul;17(7):363-71. doi: 10.1016/j.molmed.2011.02.006. Epub 2011 Apr 1. Trends Mol Med. 2011. PMID: 21459041 Review.
Cited by
-
Major differences among chemopreventive organoselenocompounds in the sustained elevation of cytoprotective genes.J Biochem Mol Toxicol. 2012 Sep;26(9):344-53. doi: 10.1002/jbt.21427. Epub 2012 Jul 16. J Biochem Mol Toxicol. 2012. PMID: 22807314 Free PMC article.
-
Thioredoxin interacting protein promotes endothelial cell inflammation in response to disturbed flow by increasing leukocyte adhesion and repressing Kruppel-like factor 2.Circ Res. 2012 Feb 17;110(4):560-8. doi: 10.1161/CIRCRESAHA.111.256362. Epub 2012 Jan 19. Circ Res. 2012. PMID: 22267843 Free PMC article.
-
Aging does not enhance experimental cigarette smoke-induced COPD in the mouse.PLoS One. 2013 Aug 2;8(8):e71410. doi: 10.1371/journal.pone.0071410. Print 2013. PLoS One. 2013. PMID: 23936505 Free PMC article.
-
Tobacco smoke induced COPD/emphysema in the animal model-are we all on the same page?Front Physiol. 2013 May 15;4:91. doi: 10.3389/fphys.2013.00091. eCollection 2013. Front Physiol. 2013. PMID: 23720629 Free PMC article.
-
Oxidative damage to nucleic acids in severe emphysema.Chest. 2009 Apr;135(4):965-974. doi: 10.1378/chest.08-2257. Epub 2008 Dec 31. Chest. 2009. PMID: 19118262 Free PMC article.
References
-
- Kluchova Z, Petrasova D, Joppa P, Dorkova Z, Tkacova R. The association between oxidative stress and obstructive lung impairment in patients with COPD. Physiol Res 2007;56:51–56. - PubMed
-
- Nadeem A, Raj HG, Chhabra SK. Increased oxidative stress and altered levels of antioxidants in chronic obstructive pulmonary disease. Inflammation 2005;29:23–32. - PubMed
-
- Ochs-Balcom HM, Grant BJ, Muti P, Sempos CT, Freudenheim JL, Browne RW, McCann SE, Trevisan M, Cassano PA, Iacoviello L, et al. Antioxidants, oxidative stress, and pulmonary function in individuals diagnosed with asthma or COPD. Eur J Clin Nutr 2006;60:991–999. - PubMed
-
- Jaiswal AK. Nrf2 signaling in coordinated activation of antioxidant gene expression. Free Radic Biol Med 2004;36:1199–1207. - PubMed
-
- Lee JM, Johnson JA. An important role of Nrf2-ARE pathway in the cellular defense mechanism. J Biochem Mol Biol 2004;37:139–143. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

