Tyrosine phosphatase SHP-1 in oxidative stress and development of allergic airway inflammation
- PMID: 18441283
- PMCID: PMC2551702
- DOI: 10.1165/rcmb.2007-0229OC
Tyrosine phosphatase SHP-1 in oxidative stress and development of allergic airway inflammation
Abstract
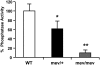
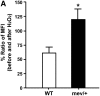
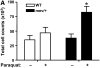
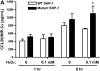
Oxidative stress has been implicated in allergic responses. SHP-1 is a target of oxidants and has been reported as a negative regulator in a mouse model of asthma. We investigated the effect of oxidative stress on the development of allergic airway inflammation in heterozygous viable motheaten (mev/+) mice deficient of SHP-1. Wild-type (WT) and mev/+ mice were compared in this study. Human alveolar epithelial cells (A549) transfected with mutant SHP-1 gene were used to evaluate the role of SHP-1 in lung epithelial cells. Hydrogen peroxide (H(2)O(2)) and Paraquat were used in vitro and in vivo, respectively. We also investigated whether mev/+ mice can break immune tolerance when exposed to aeroallergen intranasally. Compared with WT mice, bronchoalveolar lavage (BAL) cells and splenocytes from mev/+ mice showed a different response to oxidant stress. This includes a significant enhancement of intracellular reactive oxygen species and STAT6 phosphorylation in vitro and increased CCL20, decreased IL-10, and increased number of dendritic cells in BAL fluid in vivo. Mutant SHP-1-transfected epithelial cells secreted higher levels of CCL20 and RANTES after exposure to oxidative stress. Furthermore, break of immune tolerance, as development of allergic airway inflammation, was observed in mev/+ mice after allergen exposure, which was suppressed by antioxidant N-acetylcystein. These data suggest that SHP-1 plays an important role in regulating oxidative stress. Thus, increased intracellular oxidative stress and lack of SHP-1 in the presence of T helper cell type 2-prone cellular activation may lead to the development of allergic airway inflammation.
Figures








Similar articles
-
SHP-1 regulation of mast cell function in allergic inflammation and anaphylaxis.PLoS One. 2013;8(2):e55763. doi: 10.1371/journal.pone.0055763. Epub 2013 Feb 4. PLoS One. 2013. PMID: 23390550 Free PMC article.
-
Clusterin Modulates Allergic Airway Inflammation by Attenuating CCL20-Mediated Dendritic Cell Recruitment.J Immunol. 2016 Mar 1;196(5):2021-30. doi: 10.4049/jimmunol.1500747. Epub 2016 Jan 29. J Immunol. 2016. PMID: 26826245
-
SHP-1 deficient mast cells are hyperresponsive to stimulation and critical in initiating allergic inflammation in the lung.J Immunol. 2010 Feb 1;184(3):1180-90. doi: 10.4049/jimmunol.0901972. Epub 2009 Dec 30. J Immunol. 2010. PMID: 20042576 Free PMC article.
-
The tyrosine phosphatase, SHP-1, is involved in bronchial mucin production during oxidative stress.Biochem Biophys Res Commun. 2010 Feb 26;393(1):137-43. doi: 10.1016/j.bbrc.2010.01.102. Epub 2010 Feb 1. Biochem Biophys Res Commun. 2010. PMID: 20117097
-
Tyrosine phosphatase SHP-1 in allergic and anaphylactic inflammation.Immunol Res. 2010 Jul;47(1-3):3-13. doi: 10.1007/s12026-009-8134-5. Immunol Res. 2010. PMID: 20077161 Free PMC article. Review.
Cited by
-
SHP-1 regulation of mast cell function in allergic inflammation and anaphylaxis.PLoS One. 2013;8(2):e55763. doi: 10.1371/journal.pone.0055763. Epub 2013 Feb 4. PLoS One. 2013. PMID: 23390550 Free PMC article.
-
The role of oxidative stress in the pathogenesis of asthma.Allergy Asthma Immunol Res. 2010 Jul;2(3):183-7. doi: 10.4168/aair.2010.2.3.183. Epub 2010 Apr 29. Allergy Asthma Immunol Res. 2010. PMID: 20592917 Free PMC article.
-
Pesticides are Associated with Allergic and Non-Allergic Wheeze among Male Farmers.Environ Health Perspect. 2017 Apr;125(4):535-543. doi: 10.1289/EHP315. Epub 2016 Jul 6. Environ Health Perspect. 2017. PMID: 27384423 Free PMC article.
-
SHP-1 as a critical regulator of Mycoplasma pneumoniae-induced inflammation in human asthmatic airway epithelial cells.J Immunol. 2012 Apr 1;188(7):3371-81. doi: 10.4049/jimmunol.1100573. Epub 2012 Feb 27. J Immunol. 2012. PMID: 22371396 Free PMC article.
-
Commentary: IL-4 and IL-13 receptors and signaling.Cytokine. 2015 Sep;75(1):38-50. doi: 10.1016/j.cyto.2015.05.023. Epub 2015 Jul 14. Cytokine. 2015. PMID: 26187331 Free PMC article.
References
-
- Kelly FJ, Mudway I, Blomberg A, Frew A, Sandstrom T. Altered lung antioxidant status in patients with mild asthma. Lancet 1999;354:482–483. - PubMed
-
- Nadeem A, Chhabra SK, Masood A, Raj HG. Increased oxidative stress and altered levels of antioxidants in asthma. J Allergy Clin Immunol 2003;111:72–78. - PubMed
-
- Rahman I, Morrison D, Donaldson K, MacNee W. Systemic oxidative stress in asthma, COPD, and smokers. Am J Respir Crit Care Med 1996;154:1055–1060. - PubMed
-
- Ercan H, Birben E, Dizdar EA, Keskin O, Karaaslan C, Soyer OU, Dut R, Sackesen C, Besler T, Kalayci O. Oxidative stress and genetic and epidemiologic determinants of oxidant injury in childhood asthma. J Allergy Clin Immunol 2006;118:1097–1104. - PubMed
-
- Rahman I, Yang SR, Biswas SK. Current concepts of redox signaling in the lungs. Antioxid Redox Signal 2006;8:681–689. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

