Hyaluronan-CD44 interaction activates stem cell marker Nanog, Stat-3-mediated MDR1 gene expression, and ankyrin-regulated multidrug efflux in breast and ovarian tumor cells
- PMID: 18441325
- PMCID: PMC2427357
- DOI: 10.1074/jbc.M800109200
Hyaluronan-CD44 interaction activates stem cell marker Nanog, Stat-3-mediated MDR1 gene expression, and ankyrin-regulated multidrug efflux in breast and ovarian tumor cells
Abstract
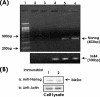
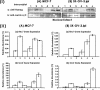
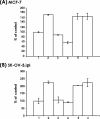
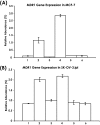
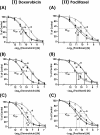
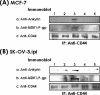
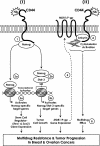
Hyaluronan (HA) is a major glycosaminoglycan in the extracellular matrix whose expression is tightly linked to multidrug resistance and tumor progression. In this study we investigated HA-induced interaction between CD44 (a HA receptor) and Nanog (an embryonic stem cell transcription factor) in both human breast tumor cells (MCF-7 cells) and human ovarian tumor cells (SK-OV-3.ipl cells). Using a specific primer pair to amplify Nanog by reverse transcriptase-PCR, we detected the expression of Nanog transcript in both tumor cell lines. In addition, our results reveal that HA binding to these tumor cells promotes Nanog protein association with CD44 followed by Nanog activation and the expression of pluripotent stem cell regulators (e.g. Rex1 and Sox2). Nanog also forms a complex with the "signal transducer and activator of transcription protein 3" (Stat-3) in the nucleus leading to Stat-3-specific transcriptional activation and multidrug transporter, MDR1 (P-glycoprotein) gene expression. Furthermore, we observed that HA-CD44 interaction induces ankyrin (a cytoskeletal protein) binding to MDR1 resulting in the efflux of chemotherapeutic drugs (e.g. doxorubicin and paclitaxel (Taxol)) and chemoresistance in these tumor cells. Overexpression of Nanog by transfecting tumor cells with Nanog cDNA stimulates Stat-3 transcriptional activation, MDR1 overexpression, and multidrug resistance. Down regulation of Nanog signaling or ankyrin function (by transfecting tumor cells with Nanog small interfering RNA or ankyrin repeat domain cDNA) not only blocks HA/CD44-mediated tumor cell behaviors but also enhances chemosensitivity. Taken together, these findings suggest that targeting HA/CD44-mediated Nanog-Stat-3 signaling pathways and ankyrin/cytoskeleton function may represent a novel approach to overcome chemotherapy resistance in some breast and ovarian tumor cells displaying stem cell marker properties during tumor progression.
Figures



Similar articles
-
Hyaluronan-CD44 interaction with protein kinase C(epsilon) promotes oncogenic signaling by the stem cell marker Nanog and the Production of microRNA-21, leading to down-regulation of the tumor suppressor protein PDCD4, anti-apoptosis, and chemotherapy resistance in breast tumor cells.J Biol Chem. 2009 Sep 25;284(39):26533-46. doi: 10.1074/jbc.M109.027466. Epub 2009 Jul 24. J Biol Chem. 2009. PMID: 19633292 Free PMC article.
-
Stem cell marker (Nanog) and Stat-3 signaling promote MicroRNA-21 expression and chemoresistance in hyaluronan/CD44-activated head and neck squamous cell carcinoma cells.Oncogene. 2012 Jan 12;31(2):149-60. doi: 10.1038/onc.2011.222. Epub 2011 Jun 20. Oncogene. 2012. PMID: 21685938 Free PMC article.
-
Hyaluronan-mediated CD44 interaction with p300 and SIRT1 regulates beta-catenin signaling and NFkappaB-specific transcription activity leading to MDR1 and Bcl-xL gene expression and chemoresistance in breast tumor cells.J Biol Chem. 2009 Jan 30;284(5):2657-2671. doi: 10.1074/jbc.M806708200. Epub 2008 Dec 1. J Biol Chem. 2009. PMID: 19047049 Free PMC article.
-
Activation of Matrix Hyaluronan-Mediated CD44 Signaling, Epigenetic Regulation and Chemoresistance in Head and Neck Cancer Stem Cells.Int J Mol Sci. 2017 Aug 24;18(9):1849. doi: 10.3390/ijms18091849. Int J Mol Sci. 2017. PMID: 28837080 Free PMC article. Review.
-
Hyaluronan-CD44 interaction promotes oncogenic signaling, microRNA functions, chemoresistance, and radiation resistance in cancer stem cells leading to tumor progression.Adv Cancer Res. 2014;123:255-75. doi: 10.1016/B978-0-12-800092-2.00010-1. Adv Cancer Res. 2014. PMID: 25081533 Free PMC article. Review.
Cited by
-
Sphere-forming tumor cells possess stem-like properties in human fibrosarcoma primary tumors and cell lines.Oncol Lett. 2012 Dec;4(6):1315-1320. doi: 10.3892/ol.2012.940. Epub 2012 Sep 26. Oncol Lett. 2012. PMID: 23205129 Free PMC article.
-
v-Src Oncogene Induces Trop2 Proteolytic Activation via Cyclin D1.Cancer Res. 2016 Nov 15;76(22):6723-6734. doi: 10.1158/0008-5472.CAN-15-3327. Epub 2016 Sep 15. Cancer Res. 2016. PMID: 27634768 Free PMC article.
-
Nanog increases focal adhesion kinase (FAK) promoter activity and expression and directly binds to FAK protein to be phosphorylated.J Biol Chem. 2012 May 25;287(22):18656-73. doi: 10.1074/jbc.M111.322883. Epub 2012 Apr 5. J Biol Chem. 2012. PMID: 22493428 Free PMC article.
-
High Levels of Hyaluronic Acid Synthase-2 Mediate NRF2-Driven Chemoresistance in Breast Cancer Cells.Biomol Ther (Seoul). 2022 Jul 1;30(4):368-379. doi: 10.4062/biomolther.2022.074. Biomol Ther (Seoul). 2022. PMID: 35768333 Free PMC article.
-
[Mechanism of STAT3 phosphorylation mediated leukemia cells resistance to doxorubicin].Zhonghua Xue Ye Xue Za Zhi. 2020 Jan 14;41(1):69-71. doi: 10.3760/cma.j.issn.0253-2727.2020.01.013. Zhonghua Xue Ye Xue Za Zhi. 2020. PMID: 32023758 Free PMC article. Chinese. No abstract available.
References
-
- Harnett, P. R., Kirsten, F., and Tattersall, M. H. (1986) J. Clin. Oncol. 4 952-957 - PubMed
-
- Mollinedo, F. (2005) IDrugs 8 127-143 - PubMed
-
- Hehlgans, S., Haase, M., and Cordes, N. (2007) Biochim. Biophys. Acta 1775 163-180 - PubMed
-
- Nishio, K., and Saijo, N. (1999) Anticancer Drug Des. 14 133-141 - PubMed
-
- Laurent, T. C., and Fraser, J. R. E. (1992) FASEB J. 6 2397-2404 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

