Expression of the beta-oxidation gene 3-ketoacyl-CoA thiolase 2 (KAT2) is required for the timely onset of natural and dark-induced leaf senescence in Arabidopsis
- PMID: 18441338
- PMCID: PMC2413277
- DOI: 10.1093/jxb/ern079
Expression of the beta-oxidation gene 3-ketoacyl-CoA thiolase 2 (KAT2) is required for the timely onset of natural and dark-induced leaf senescence in Arabidopsis
Abstract
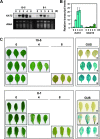
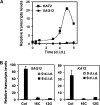
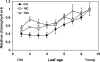
The onset of leaf senescence is regulated by a complex mechanism involving positive and negative regulators. Among positive regulators, jasmonic acid (JA) accumulates in senescing leaves and the JA-insensitive coi1-1 mutant displays delayed leaf senescence in Arabidopsis. A strong activated expression of the gene coding for the JA-biosynthetic beta-oxidation enzyme 3-ketoacyl-CoA thiolase 2 (KAT2) in natural and dark-induced senescing leaves of Arabidopsis thaliana is reported here. By using KAT2::GUS and KAT2::LUC transgenic plants, it was observed that dark-induced KAT2 activation occurred both in excised leaves as well as in whole darkened plants. The KAT2 activation associated with dark-induced senescence occurred soon after a move to darkness, and it preceded the detection of symptoms and the expression of senescence-associated gene (SAG) markers. Transgenic plants with reduced expression of the KAT2 gene showed a significant delayed senescence both in natural and dark-induced processes. The rapid induction of the KAT2 gene in senescence-promoting conditions as well as the delayed senescence phenotype and the reduced SAG expression in KAT2 antisense transgenic plants, point to KAT2 as an essential component for the timely onset of leaf senescence in Arabidopsis.
Figures
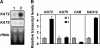


Similar articles
-
The Arabidopsis 3-ketoacyl-CoA thiolase-2 (kat2-1) mutant exhibits increased flowering but reduced reproductive success.J Exp Bot. 2007;58(11):2959-68. doi: 10.1093/jxb/erm146. Epub 2007 Aug 28. J Exp Bot. 2007. PMID: 17728299
-
Requirement for 3-ketoacyl-CoA thiolase-2 in peroxisome development, fatty acid beta-oxidation and breakdown of triacylglycerol in lipid bodies of Arabidopsis seedlings.Plant J. 2001 Oct;28(1):1-12. doi: 10.1046/j.1365-313x.2001.01095.x. Plant J. 2001. PMID: 11696182
-
Evidence supporting a role of jasmonic acid in Arabidopsis leaf senescence.Plant Physiol. 2002 Mar;128(3):876-84. doi: 10.1104/pp.010843. Plant Physiol. 2002. PMID: 11891244 Free PMC article.
-
Arabidopsis 3-ketoacyl-CoA thiolase-2 (KAT2), an enzyme of fatty acid β-oxidation, is involved in ABA signal transduction.Plant Cell Physiol. 2011 Mar;52(3):528-38. doi: 10.1093/pcp/pcr008. Epub 2011 Jan 20. Plant Cell Physiol. 2011. PMID: 21257607
-
Knockout of the two evolutionarily conserved peroxisomal 3-ketoacyl-CoA thiolases in Arabidopsis recapitulates the abnormal inflorescence meristem 1 phenotype.J Exp Bot. 2014 Dec;65(22):6723-33. doi: 10.1093/jxb/eru397. Epub 2014 Oct 7. J Exp Bot. 2014. PMID: 25297549 Free PMC article.
Cited by
-
Differential impact of lipoxygenase 2 and jasmonates on natural and stress-induced senescence in Arabidopsis.Plant Physiol. 2010 Apr;152(4):1940-50. doi: 10.1104/pp.110.153114. Epub 2010 Feb 26. Plant Physiol. 2010. PMID: 20190093 Free PMC article.
-
Turnover of fatty acids during natural senescence of Arabidopsis, Brachypodium, and switchgrass and in Arabidopsis beta-oxidation mutants.Plant Physiol. 2009 Aug;150(4):1981-9. doi: 10.1104/pp.109.140491. Epub 2009 Jun 26. Plant Physiol. 2009. PMID: 19561121 Free PMC article.
-
Ring/U-Box Protein AtUSR1 Functions in Promoting Leaf Senescence Through JA Signaling Pathway in Arabidopsis.Front Plant Sci. 2020 Dec 16;11:608589. doi: 10.3389/fpls.2020.608589. eCollection 2020. Front Plant Sci. 2020. PMID: 33391323 Free PMC article.
-
Chemical Genetics Approach Identifies Abnormal Inflorescence Meristem 1 as a Putative Target of a Novel Sulfonamide That Protects Catalase2-Deficient Arabidopsis against Photorespiratory Stress.Cells. 2020 Sep 2;9(9):2026. doi: 10.3390/cells9092026. Cells. 2020. PMID: 32887516 Free PMC article.
-
Cloning, expression and purification of an acetoacetyl CoA thiolase from sunflower cotyledon.Int J Biol Sci. 2009 Dec 2;5(7):736-44. doi: 10.7150/ijbs.5.736. Int J Biol Sci. 2009. PMID: 20011134 Free PMC article.
References
-
- Afitlhile MM, Fukushige H, Nishimura M, Hildebrand DF. A defect in glyoxysomal fatty acid beta-oxidation reduces jasmonic acid accumulation in Arabidopsis. Plant Physiology and Biochemistry. 2005;43:603–609. - PubMed
-
- Baker A, Graham IA, Holdsworth M, Smith SM, Theodoulou FL. Chewing the fat: beta-oxidation in signalling and development. Trends in Plant Science. 2006;11:124–32. - PubMed
-
- Buchanan-Wollaston V, Earl S, Harrison E, Mathas E, Navabpour S, Page T, Pink D. The molecular analysis of leaf senescence: a genomics approach. Plant Biotechnology Journal. 2003;1:3–22. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

