Nortriptyline plus nicotine replacement versus placebo plus nicotine replacement for smoking cessation: pragmatic randomised controlled trial
- PMID: 18441375
- PMCID: PMC2405820
- DOI: 10.1136/bmj.39545.852616.BE
Nortriptyline plus nicotine replacement versus placebo plus nicotine replacement for smoking cessation: pragmatic randomised controlled trial
Abstract
Objective: To test the efficacy of nortriptyline plus nicotine replacement therapy compared with placebo plus nicotine replacement therapy for smoking cessation.
Design: Pragmatic randomised controlled trial.
Setting: National Health Service stop smoking service clinics.
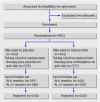
Participants: 901 people trying to stop smoking.
Interventions: Participants chose their nicotine replacement product, including combinations of nicotine replacement therapy, and received behavioural support. Nortriptyline was started one to two weeks before quit day, with the dose increased from 25 mg to 75 mg daily for eight weeks and reduced if not tolerated.
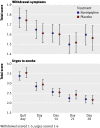
Main outcome measures: Primary outcome was prolonged confirmed abstinence at six months. Secondary outcomes were prolonged abstinence at 12 months, drug use, severity of side effects, nicotine withdrawal symptoms, and urges to smoke.
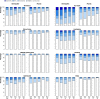
Results: 72 of 445 (16%) people using nortriptyline and 55 of 456 (12%) using placebo achieved prolonged abstinence at six months (relative risk 1.34, 95% confidence interval 0.97 to 1.86). At 12 months the corresponding values were 49 (11%) for nortriptyline and 40 (9%) for placebo (1.26, 0.84 to 1.87). 337 (79%) people in the nortriptyline arm and 325 (75%) in the placebo arm were taking combination treatment on quit day, median 75 mg per day in both groups. More people in the nortriptyline arm than in the placebo arm took lower doses. The nortriptyline arm had noticeably higher severity ratings for dry mouth and constipation than the placebo arm, with slightly higher ratings for sweating and feeling shaky. Both groups had similar urges to smoke, but nortriptyline reduced depression and anxiety. Overall, withdrawal symptom scores did not differ.
Conclusions: Nortriptyline and nicotine replacement therapy are both effective for smoking cessation but the effect of the combination is less than either alone and evidence is lacking that combination treatment is more effective than either alone. Trial registration Current Controlled Trials ISRCTN57852484.
Conflict of interest statement
Competing interests: PA has done consultancy work for the pharmaceutical and biotechnology industry that has led to payments to him and his institution. This includes work for companies providing smoking cessation treatment, including nicotine replacement therapy. MM has received consultancy income from the European Network for Smoking Prevention and has provided scientific consultancy services through the University of Oxford ISIS Innovation to the National Audit Office and G-Nostics.
Ethical approval: We obtained approval from the multicentre research ethics committee and all local research ethics committees for the areas in which our trial took place. We obtained a clinical trials authorisation from the Medicines and Healthcare products Regulatory Agency. We obtained approval from all NHS research and development offices of the primary care organisations for the areas in which our trial took place.
Figures

Comment in
-
Smoking cessation in primary care.BMJ. 2008 May 31;336(7655):1200-1. doi: 10.1136/bmj.39546.520694.80. Epub 2008 Apr 27. BMJ. 2008. PMID: 18441374 Free PMC article.
References
-
- West R. The clinical significance of ‘small’ effects of smoking cessation treatments. Addiction 2007;102:506-9. - PubMed
-
- Cahill K, Stead LF, Lancaster T. Nicotine receptor partial agonists for smoking cessation. Cochrane Database Syst Rev 2007;(1):CD006103 pub2. - PubMed
-
- Hughes JR, Stead LF, Lancaster T. Antidepressants for smoking cessation. Cochrane Database Syst Rev 2007;(1):CD000031 pub3. - PubMed
-
- Stead LF, Perera R, Bullen C, Mant D, Lancaster T. Nicotine replacement for smoking cessation. Cochrane Database Syst Rev 2008;(1):CD000146 pub3. - PubMed
-
- Hughes JR. Effects of abstinence from tobacco: valid symptoms and time course. Nicotine Tob Res 2007;9:315-27. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
